Summary
Simvastatin in addition to usual care did not reduce the rate of or time to exacerbations in patients with moderate to severe chronic obstructive pulmonary disease (COPD) in the Simvastatin Therapy for Moderate and Severe COPD study [STATCOPE; NCT01061671; Criner GJ et al. N Engl J Med 2014]. This multicenter study conducted in the United States and Canada and sponsored by respective federal agencies sought to validate benefits seen with statins, perhaps related to their anti-inflammatory effects, in retrospective studies of COPD.
- Chronic Obstructive Pulmonary Disease Pulmonary Clinical Trials
- Lipid Disorders
- Chronic Obstructive Pulmonary Disease
- Pulmonary & Critical Care
- Pulmonary Clinical Trials
- Lipid Disorders
Simvastatin in addition to usual care did not reduce the rate of or time to exacerbations in patients with moderate to severe chronic obstructive pulmonary disease (COPD) in the Simvastatin Therapy for Moderate and Severe COPD study [STATCOPE; NCT01061671; Criner GJ et al. N Engl J Med 2014]. This multicenter study conducted in the United States and Canada and sponsored by respective federal agencies sought to validate benefits seen with statins, perhaps related to their anti-inflammatory effects, in retrospective studies of COPD, stated Gerard J. Criner, MD, Temple University, Philadelphia, Pennsylvania, USA. The study was terminated early for futility.
In total, 885 patients were randomized to simvastatin 40 mg/day (n=430) or placebo (n=329) on top of usual care for COPD; 74% of each group completed the 12-month follow-up, and 20% of the simvastatin and 18% of the placebo groups completed the 36-month follow-up. The patients (44% women) had a mean age of 62 years, a mean smoking history of 50.6 pack-years, and a mean forced expiratory volume in 1 second of 41.6% of the predicted value. Key exclusion criteria were the presence of diabetes or cardiovascular disease, need for statins according to the Adult Treatment Panel III criteria, and treatment with amlodipine, verapamil, diltiazem, or ranolazine.
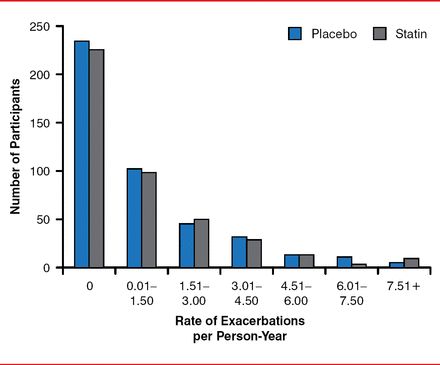
The primary outcome, mean number of COPD exacerbations per patient per year, was similar: 1.36 with simvastatin (95% CI, 1.20–1.51) versus 1.39 with placebo (95% CI, 1.22–1.54; p=0.54). The number of exacerbations per person-year is shown in Figure 1. The median time to first exacerbation was 223 days with simvastatin (95% CI, 195–275 days), compared with 231 days with placebo (95% CI, 193–303 days; p=0.34). There was no difference found in the severity of COPD exacerbations with simvastatin compared with placebo, with similar rates of mild, moderate, severe, and very severe exacerbations. No difference in the mean exacerbation rates was found with simvastatin compared with placebo for any prespecified subgroups, including smoking status, study center, gender, and race.
Mean Number of Exacerbations Per Person-Year
Reproduced with permission from GJ Criner, MD.
There was no difference between simvastatin and placebo for the secondary endpoints of change in spirometric measures of lung function and quality of life, as assessed by the St. George's Respiratory Questionnaire. The rates of nonfatal serious adverse events (SAEs) were similar (0.63 and 0.62 per participant-year with simvastatin and placebo, respectively), but the overall number of SAEs was limited.
Simvastatin was associated with improvements in total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, and high-density lipoprotein cholesterol (HDL-C). Baseline lipid levels were similar in study participants. LDL-C was decreased by 33.2 mg/dL with simvastatin and 6.6 mg/dL with placebo, and HDL-C was increased by 2.5 mg/dL and decreased by 0.5 mg/dL, respectively.
Study limitations included not using C-reactive protein level as an inclusion criterion and restriction of the patient population to those with moderate or severe COPD.
The STATCOPE trial did not demonstrate a therapeutic benefit, from a respiratory perspective, of statins in patients with moderate to severe COPD, stated Dr. Criner.
- © 2014 MD Conference Express®