Summary
This article addresses the just-published guidelines of the American Thoracic Society and European Respiratory Society concerning the evaluation, diagnosis, and treatment of severe asthma [Chung KF et al. Eur Respir J 2014].
- Asthma
- Pulmonary Guidelines
- Pulmonary & Respiratory Medicine
A scientific symposium addressed the just-published guidelines of the American Thoracic Society and European Respiratory Society concerning the evaluation, diagnosis, and treatment of severe asthma [Chung KF et al. Eur Respir J 2014].
Sally Wenzel, MD, University of Pittsburgh, Pittsburgh, Pennsylvania, USA, discussed the guidelines concerning the transition from mild to severe asthma and related diagnostic considerations. At the core of the guidelines is the distinction between severe and mild asthma. Severe asthma is defined in a 3-step process. Step 1 confirms an asthma diagnosis and identifies difficult-to-treat asthma. This step requires the exclusion of other (primary) diagnoses, such as vocal cord dysfunction, and treatment of comorbidities including poor adherence and environmental exposure sources that can be modified, and it should include evaluation and treatment by an asthma specialist for ≥3 months.
Step 2 differentiates severe from milder asthma. This step involves determining whether patients require high-dose inhaled (or systemic) corticosteroids to maintain control of their asthma or whether their asthma is not controlled despite this therapy. Assessment of comorbidities and factors that can worsen asthma (or make it appear worse than it is) is also important (Table 1).
Comorbidities and Contributing Factors to Assess
Step 3 determines whether severe asthma is controlled or uncontrolled (Table 2).
Criteria for Severe Uncontrolled Asthmaa
When these criteria are not met but a patient has asthma that becomes worse when corticosteroid treatment is altered, the asthma is also considered severe. In many cases today, patients with severe asthma are not fully controlled, even with the use of high-dose inhaled corticosteroids (≥1000 mg fluticasone propionate or equivalent) along with a second controller. Following these 3 steps is critical, as up to one-third of patients with diagnosed asthma or severe asthma may not have asthma. Recognizing this reality, the guidelines support (but have no recommendations for) an approach to severe asthma that is practical and based on clinical sense. When confronted by asthma with symptoms and spirometric results that are atypical of asthma, additional testing may be necessary on a case-by-case basis. This testing can include methacholine challenge, diffusion capacity for carbon monoxide, laryngoscopy, determination of immunoglobulin E and antineutrophil cytoplasmic antibody, autoimmune evaluation, and even echocardiography. The guidelines do address the use of chest high-resolution computed tomography (HRCT) in patients with symptoms of severe asthma without specific indications for the procedure on the basis of history, symptoms, and/or prior results. Chest HRCT is recommended only when the asthma appears atypical (eg, excessive mucus, rapid decline in lung function including carbon monoxide transfer, absence of atopy in a child with difficult asthma).
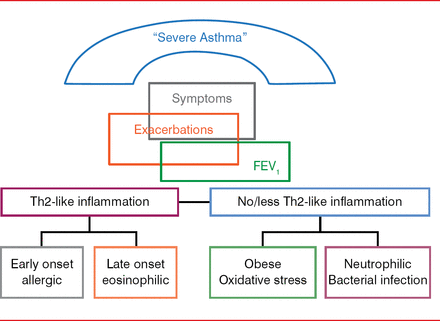
Despite this definition, severe asthma includes a spectrum of phenotypes. Emerging studies suggest that even using rigorous definitions such as this, some patients with severe asthma may be missed, who by traditional approaches are considered to have relatively mild asthma [Moore WC et al. Am J Respir Crit Care Med 2010]. The guidelines also consider approaches that can be useful in assessing asthma phenotypes, such as age at onset (early onset more likely atopic or allergic, later onset more varied), biomarkers [Pavord ID et al. Lancet 2012; Haldar P et al. N Engl J Med 2009], and outcomes based on phenotypic evaluations including the absence or presence of Th2-like inflammation. The latter 2 approaches are in their infancy, with much research yet to be done. Research to date supports the presence of various severe asthma phenotypes related to the Th2-like inflammation (Figure 1).
Potential Severe Asthma Phenotypes
FEV1=forced expiratory volume in 1 second.
Adapted from Wenzel S. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012;18(5):716–725.
The guidelines address the issue of whether treatment of adults with severe asthma should be guided by sputum eosinophil count, instead of only clinical criteria. The guidelines' conditional recommendation is that these patients be treated on the basis of both approaches, not just clinical criteria. The guidelines recognize the lack of a standard measurement of sputum eosinophils and suggest that the dual approach be used only at centers with experience in the measurement of sputum eosinophils and for patients who can produce sputum.
The use of fractional exhaled nitric oxide (FeNO) in addition to clinical criteria as a guide to treatment is not recommended for children and adults with severe asthma, given the cost involved and the inability of FeNO to distinguish mild from severe asthma.
Looking ahead, as in other fields, the use of genotyping is showing potential in distinguishing mild and moderate asthma from severe asthma; however, it will be some time before this approach has practical applications.
Kian Fan Chung, MD, PhD, Royal Brompton Hospital, London, United Kingdom, discussed treatment recommendations for anti–immunoglobulin E therapy using omalizumab and bronchial thermoplasty.
Evidence for anti–immunoglobulin E therapy came from 8 randomized controlled trials (6 in adolescents and adults, 2 in children). Collectively, the evidence demonstrated improved quality of life (risk ratio [RR], 1.19; 95% CI, 1.08 to 1.30), decreased use of oral steroids (RR, 0.73; 95% CI, 0.56 to 0.94), and lessened exacerbation of asthma (RR, 0.72. 95% CI, 0.59 to 0.86) in subjects receiving omalizumab. The treatment was also associated with reduced death in 3 studies, hospitalization in 1 study, and any or serious adverse events in 6 studies, with a calculated 0.09% risk for anaphylaxis. The data prompted the recommendation (conditional, on the basis of low-quality or very low quality evidence) for the use of omalizumab in select children and adults with severe asthma (Table 3).
Who Should Receive Omalizumab?
The recommendation of selected treatment is supported by results of the Omalizumab (Xolair) in Subjects With Moderate to Severe Persistent Asthma study [EXTRA] of 850 patients with uncontrolled, severe, persistent asthma, which demonstrated beneficial responses to omalizumab in subjects with elevated baseline levels of FeNO, blood eosinophils, and serum periostin [Hanania NA et al. Ann Intern Med 2011].
Bronchial thermoplasty is a US Food and Drug Administration–approved technique that relies on radiofrequency energy to heat airway tissue, with the aim of destroying airway smooth muscle (which cannot regenerate) as a means of alleviating severe asthma. Ample evidence of success of bronchial thermoplasty has come from several studies involving >1500 patients, some of whom were followed up for 5 years [Thomson NC et al. BMC Pulmon Med 2011; Castro M et al. Am J Respir Crit Care Med 2010; Cox G et al. N Engl J Med 2007; Pavord ID et al. Am J Respir Crit Care Med 2007]. The procedure favorably affects quality of life (test for overall effect, z=2.16, p<0.0001) and, although not significantly, asthma control (test for overall effect, z=1.54, p=0.12).
Side effects of bronchial thermoplasty include wheezing, cough, dyspnea, sputum production, thoracic discomfort, fever, and sleep disturbance. But these symptoms develop immediately after the procedure and are transient. They disappear within a week after the procedure, with no further adverse events or complications developing over time in the one follow-up study conducted to date that examined long-term adverse events [Wechsler et al. J Allergy Clin Immunol 2013].
The procedure is recommended for use in the treatment of patients with severe asthma, with the caveat that the nature of the radiofrequency effect still needs to be precisely determined, as does the cost-effectiveness.
Peter G. Gibson, MBBS, University of Newcastle, Newcastle, Australia, discussed the guideline recommendations pertaining to methotrexate, macrolides, and antifungal agents. The question of whether methotrexate should be used in the treatment of patients with severe asthma has been pondered in light of the observations that methotrexate reverses corticosteroid insensitivity and prednisolone-mediated suppression of blood T-cell proliferation. As well, it is debatable whether macrolide antibiotics, including troleandomycin, clarithromycin, and azithromycin, should be used in these patients. Macrolide antibiotics do have proven efficacy as antibacterial and anti-inflammatory agents in severe asthma, and, in the case of troleandomycin, they inhibit corticosteroid metabolism, making the drug more available for therapeutic use. Yet the evidence comes from a small number of studies involving relatively few patients. So, despite the apparent efficacy of macrolides, the guideline recommendation is for physicians not to use these antibiotics in the treatment of severe asthma. Issues including clinical benefit and the development of antibiotic resistance need to be studied further.
Concerning antifungal agents, colonization of the airway by Aspergillus fumigatus in severe asthma is a real and serious problem. Treatment with antifungal drugs has the potential to modulate acquired and innate immunity to the fungal infection, which can quell the activation of inflammatory cells and curb bronchiectasis. The double-blind, placebo-controlled, randomized controlled Effectiveness of Voriconazole in the Treatment of Aspergillus fumigatus–Associated Asthma trial [EVITA3] involving 65 patients with moderate to severe asthma failed to demonstrate a beneficial effect of a 3-month treatment for the rate of severe exacerbations or the quality of life [Agbetile J et al. J Allergy Clin Immunol 2013]. Nonetheless, the perceived benefits do argue for the use of antifungal agents in patients with severe asthma and recurrent exacerbations of allergic bronchopulmonary aspergillosis.
- © 2014 MD Conference Express®