Summary
The AMPLATZER Duct Occluder II [ADO II] device is safe and effective for the closure of small- to moderate-sized patent ductus arteriosus (PDA), according to results of the ADO II prospective, single-arm, open-label, multicenter investigational device exemption clinical trial conducted to evaluate the clinical safety and efficacy of the ADO II device [NCT00713700].
- interventional techniques & devices
- cardiology genomics
- cardiology clinical trials
The AMPLATZER Duct Occluder II [ADO II] device is safe and effective for the closure of small- to moderate-sized patent ductus arteriosus (PDA). The ADO II's small diameter, delivery systems, and device symmetry allows for a more flexible approach than ADO I and may broaden the use of this device in potential patient populations.
Daniel Gruenstein, MD, University of Minnesota Children's Hospital, Minneapolis, Minnesota, USA, presented the results of the ADO II prospective, single-arm, open-label, multicenter investigational device exemption clinical trial conducted to evaluate the clinical safety and efficacy of the ADO II device [NCT00713700].
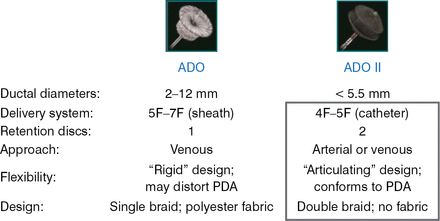
In 2006, the ADO I device was shown to be effective in the closure of medium to large PDA diameters [Wang JK et al. Int J Cardiol 2006]. The ADO II device was designed for smaller PDA diameters. Figure 1 shows the distinctive design features of the ADO I and ADO II devices.
ADO I Versus ADO II Design
ADO=AMPLATZER Duct Occluder; PDA=patent ductus arteriosus.
Reproduced with permission from D Gruenstein, MD.
The current study assessed the safety and efficacy of the ADO II device using a primary safety end point of freedom from device- and procedure-related serious adverse events at 6 months and a primary efficacy end point of an absence of residual PDA shunt at 6 months. The study compared the performance of the ADO II device against the performance goals based on the original ADO clinical trial.
A total of 192 patients met the inclusion criteria and were enrolled in the study between August 2008 and April 2011. Patients were eligible if they were aged 6 months to 18 years and weighed > 6 kg [AGA Medical Corporation. AMPLATZER Duct Occluder II Instructions for Use. St. Jude Medical. 2013]. Inclusion criteria also included an anatomy with a PDA diameter of < 5.5 mm and PDA length of 3 to 12 mm. Patients were excluded from the study if they had a descending aorta < 10 mm, a right-to-left shunt, high pulmonary vascular resistance, or medical comorbidities.
The enrolled patients had a mean age of 4.4 years and mean weight of 19.35 kg. Of the 192 patients, 178 (92.7%) were successfully implanted with the device and an attempt at implantation was done in the remaining 14 patients. Safety data at 6 months was obtained for all 192 patients, with continual follow-up for up to 5 years planned for the 178 patients successfully implanted.
The results of the study showed the ADO II device to be safe and effective at 6 months (Table 1).
Safety and Efficacy Results
The study also showed that the mean fluoroscopy time was 14 minutes (range, 2 to 89 minutes), with an average fluoroscopy time of 15.2 minutes when using an antegrade approach for device implantation, and 11.6 minutes using a retrograde approach.
According to Dr. Gruenstein, this provides preliminary evidence that the flexibility of the ADO II approach may reduce radiation exposure by reducing fluoroscopy times.
- © 2014 MD Conference Express®