Summary
This article discussez current understanding of the potential role for pharmacologic agents in bone healing, particularly those used for osteoporosis treatment, of which parathyroid hormone shows the most clinical promise.
- Bone Density & Structure Disorders
- Metabolic Bone Disease Metabolic Bone Disease
- Orthopaedics & Sports Medicine
Aurelia Nattiv, MD, University of California, Los Angeles, Los Angeles, California, USA, discussed current understanding of the potential role for pharmacologic agents in bone healing, particularly those used for osteoporosis treatment, of which parathyroid hormone (PTH) shows the most clinical promise.
New technologies to accelerate fracture healing have been investigated in animal studies for years, and there is growing clinical evidence to support the use of systemic agents to enhance fracture repair. Systemic agents for osteoporosis that improve bone mass and prevent fractures have received the most attention; most are US Food and Drug Administration (FDA)-approved for osteoporosis treatment but are off label for fracture healing, while others remain under investigation.
FDA-APPROVED OSTEOPOROSIS AGENTS AND FRACTURE HEALING Estrogen
Despite a lack of clinical data, animal studies have shown that estrogen therapy enhances fracture healing (Figure 1) [Kolios L et al. Calcif Tissue Int 2010], resulting in a callus with improved biomechanical competence and increased chondrocyte area and mineralization [Beil FT et al. J Trauma 2010].
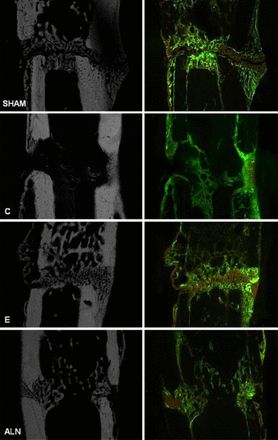
Bone Healing in a Rat Metaphyseal Fracture Model
Compared with physiologic healing in the sham group, callus formation in the osteoporotic (OP) control (C) group was enhanced but unstructured and less dense. Estrogen (E) administration induced less callus formation, but the callus present was very compact and dense, with increased amounts of trabecular structure. The alendronate (ALN) group displayed fewer and unconstrained calluses and trabeculas.
Reproduced with permission from Springer New York LLC from Kolios L, Hoerster AK, Sehmisch S, et al. Do estrogen and alendronate improve metaphyseal fracture healing when applied as osteoporosis prophylaxis? Calcif Tissue Int. 2010;86:23–32.
Raloxifene
In a rat metaphyseal tibial fracture-healing model, raloxifene markedly induced total callus formation and improved the biomechanical properties of bone healing [Stuermer EK et al. Langenbecks Arch Surg 2010].
Bisphosphonates
Although bisphosphonates (BPs) potently inhibit osteoclast-mediated bone resorption, there is mixed evidence for their effect on fracture healing. In a rat closed femur fracture model, bolus dosing with zoledronic acid increased callus size and strength without delaying endochondral ossification, although hard callus remodeling was slightly slower than in control animals [McDonald MM et al. Bone 2008].
Some of the best clinical data exist in compromised patients. In children with osteogenesis imperfecta, older age (odds ratio [OR] per year of age, 1.25; 95% CI, 1.06 to 1.47) and tibial osteotomy (OR, 3.51; 95% CI, 1.57 to 7.82) independently predicted delayed healing of lower limb fractures during pamidronate treatment. However, the treatment effect was not significant when age differences were taken into account (OR, 1.76; 95% CI, .61 to 5.10) [Munns CFJ et al. J Bone Miner Res 2004]. In a study in 19,731 older adults with humerus fractures, use of BP approximately doubled the risk for nonunion (OR, 2.37; 95% CI, 1.13 to 4.96) [Solomon DH et al. Osteoporos Int 2009].
Denosumab
Although no clinical data exist for the effect of the osteoclast inhibitor denosumab on fracture healing, it increased callus volume and bone mineral density (BMD) in a mouse femur fracture model [Gerstenfeld LC et al. J Bone Miner Res 2009]. Despite delayed callus remodeling, there was no compromise in its mechanical integrity.
PTH
PTH is the only FDA-approved anabolic therapy for osteoporosis and currently shows the most benefit for fracture healing in patients with nonunion or delayed healing. In a rat model, PTH enhanced callus formation, BMD, bone mineral content, and cartilage formation and increased mechanical strength at the tibial fracture site [Andreassen TT et al. Calcif Tissue Int 2004].
In a placebo-controlled, double-blinded, randomized, multicenter, multinational clinical study, 102 post-menopausal women with Colles' fracture who were treated conservatively without surgical intervention were randomly assigned to placebo (n = 34) or teriparatide, a recombinant form of PTH, at 20 μg (n = 34) or 40 μg (n = 34) within 10 days of fracture [Aspenberg P et al. J Bone Miner Res 2010]. Accelerated healing was seen in the teriparatide 20 μg group compared with placebo (p = .006).
In a recent prospective study in BP-associated atypical femoral fractures, 5 of 14 patients treated with 20 μg teriparatide experienced a 2- to 3-fold increase in bone remodeling markers (p = .01) and fracture healing, while 9 of 14 control patients experienced poor healing and ongoing pain [Chiang CY et al. Bone 2013].
Emerging Agents
The Wnt signaling pathway has emerged as one of the most essential signaling cascades in osteogenic disorders. Targeting this pathway can modulate fracture healing and represents an attractive therapeutic approach for the development of potential therapeutic avenues.
Romosozumab exemplifies this translation of emerging agents into clinical application. The humanized monoclonal antibody increases bone formation by binding to the osteoblast inhibitor sclerostin and was associated with increased BMD and bone formation, and decreased bone resorption, in a recent Phase 2 study in 419 post-menopausal women with low bone mass [McClung MR et al. N Engl J Med 2014].
Other agents that target the Wnt signaling pathway, such as anti-Dkk-1 antibody, remain under investigation and may soon become available for clinical use [Kim JH et al. Ther Adv Musculoskelet Dis 2013].
Future randomized controlled trials of clinical fracture healing are needed to improve the characterization of clinical end points such as decrease in pain, improved function, radiographic evidence of union, and decrease in reoperation rate.
- © 2014 MD Conference Express®