Summary
This article reports the results of the Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation—TIMI 48 trial [ENGAGE AF-TIMI 48]. This randomized, double-blind, double-dummy trial of 21,105 patients demonstrated the efficacy and safety of a once-daily regimen of the oral anticoagulant edoxaban in treatment of atrial fibrillation (AF). The primary trial results have been published [Giugliano RP et al. N Engl J Med 2013].
- Arrhythmias
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Arrhythmias
- Cardiology Clinical Trials
Robert P. Giugliano, MD, SM, Harvard Medical School, Boston, Massachusetts, USA, and senior investigator of the Thrombosis In Myocardial Infarction (TIMI) study group, reported the results of the Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-TIMI 48 trial [ENGAGE AF-TIMI 48]. This randomized, double-blind, double-dummy trial of 21,105 patients demonstrated the efficacy and safety of a once-daily regimen of the oral anticoagulant edoxaban in treatment of atrial fibrillation (AF). The primary trial results have been published [Giugliano RP et al. N Engl J Med 2013].
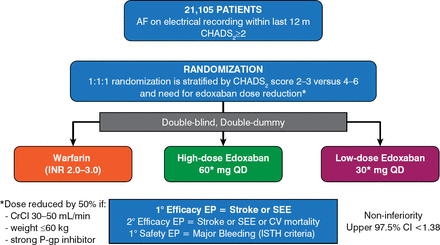
Patients with ≥ 1 confirmed episode of AF within the past 12 months prior to enrollment were randomly assigned to receive warfarin, high-dose edoxaban 60 mg once daily, or low-dose edoxaban 30 mg once daily (Figure 1).
Study Design
CrCl=creatinine clearance; EP=end point; ISTH=International Society on Thrombosis and Haemostasis; P-gp=P-glycoprotein; SEE=systemic embolic event.
Reproduced with permission from Elsevier from Ruff CT, Giugliano RP, Antman EM, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J. 2010;160(4):635–641.
The edoxaban doses were reduced from 60 to 30 mg and from 30 to 15 mg at the time of randomization for patients with creatinine clearance of 30 to 50 mL/min, those weighing ≤ 60 kg, and those who were using cardiac medications that potently inhibited P-glycoprotein, or during the study if these conditions developed. The patient completion rate was 99.5%, with only 1 patient lost during the median 2.8-year follow-up period. Analyses included primary efficacy (noninferiority) for all patients receiving ≥ 1 dose (modified intention-to-treat [ITT]) while on treatment, superiority analyses in the ITT population counting all events after randomization, and principal safety (major bleeding) in the on-treatment population.
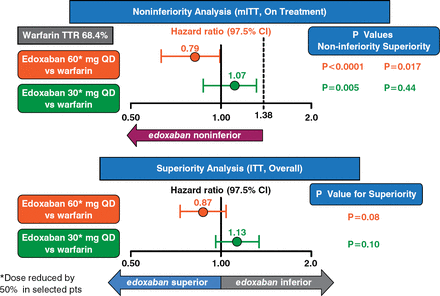
The primary end point was occurrence of stroke or systematic embolic events during follow-up. In the noninferiority modified ITT analysis, both low and high doses of edoxaban were noninferior to warfarin (p < .001 and p = .005, respectively). In the ITT analysis, neither dose of edoxaban was shown to be significantly different from warfarin (p = .08, p = .10, respectively) (Figure 2).
Primary End Point Analyses
ITT=intention-to-treat; QD=once daily; TTR=time to response.
Reproduced with permission from RP Giugliano, MD.
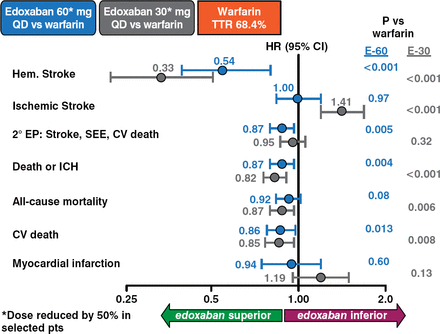
Analyses of secondary outcomes of edoxaban versus warfarin revealed superiority of edoxaban in terms of hemorrhagic stroke (both doses); secondary events including stroke, systematic embolic events, and cardiovascular death (60 mg); death or intracranial hemorrhage (both doses); all-cause mortality (30 mg); and cardiovascular death (both doses) (Figure 3).
Key Secondary Outcomes
CV=cardiovascular; EP=end point; ICH=intracranial hemorrhage; SEE=systemic embolic event.
Reproduced with permission from RP Giugliano, MD.
Both the 30- and 60-mg edoxaban doses were superior to warfarin concerning major bleeding (p < .001 for both), fatal bleeding (p < .001 and p = .006, respectively), and intracranial hemorrhage (p < .001 for both). A dose effect was more apparent for gastrointestinal bleeding, with 30 mg being superior to warfarin (p = .03) and 60 mg being inferior (p < .001). Edoxaban 30 and 60 mg was superior to warfarin in net clinical outcomes of stroke, systematic embolic events, death, and major bleeding (p < .001 and p = .003, respectively); disabling stroke, life-threatening bleeding, and death (p < .001 and p = .008, respectively); and stroke, systematic embolic events, life-threatening bleeding, and death (p = .007 and p = .003, respectively). Tolerability and the types and occurrence of adverse events were similar for warfarin and both edoxaban doses.
The frequency of hemorrhagic stroke was significantly reduced for both edoxaban doses compared with warfarin: for edoxaban 30 mg, HR .33 (95% CI, .22 to .50; p < .001), and for edoxaban 60 mg, HR .54 (95% CI, .38 to .77; p < .001). The frequency of ischemic stroke was similar between the warfarin and edoxaban 60 mg arms (p = .97) but was significantly elevated in the edoxaban 30 mg arm (p < .001).
Because transition from one anticoagulant to another is a high-risk period for patients, the study included a transition plan to protect patients during the transition period from their randomized study drug to open-label anticoagulation. The plan allowed transition to either a vitamin K antagonist (VKA) or a newer oral anticoagulant. If a VKA was selected, frequent early testing of the international normalized ratio was mandated, along with use of a VKA dosing algorithm and edoxabanplacebo transition kit (which contained matching edoxabanor placebo, depending on whether the patient had been randomly assigned to edoxaban or warfarin during the trial). There were no excess of thrombotic and bleeding events across all 3 study arms during the transition period [Ruff C et al. J Am Coll Cardiol 2014].
The trial results indicate the potential value of a once-daily edoxaban regimen, especially using 60 mg, for patients with AF.
- © 2014 MD Conference Express®