Summary
This article discusses female data from trials of patients undergoing transcatheter aortic valve replacement (TAVR). Specific trials include the ADVANCE and WIN TAVI registries, as well as the CoreValve and PARTNER trials.
- Interventional Techniques & Devices
- Valvular Disease
- Cardiology
- Interventional Techniques & Devices
- Valvular Disease
In a symposium to discuss female data from trials of patients undergoing transcatheter aortic valve replacement (TAVR), Jeffrey J. Popma, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA, discussed the outcomes of women undergoing self-expanding TAVR using the Medtronic CoreValve (MCV) prosthesis in the ADVANCE registry [NCT01074658], and the CoreValve US Extreme-Risk and CoreValve US High-Risk Pivotal Trials [NCT01240902].
According to Dr Popma, women represent approximately half of all patients undergoing TAVR in clinical trials. One thousand fifteen patients (women, n = 514) with severe aortic stenosis (AS) undergoing TAVR with use of the MCV prosthesis in 44 centers in 12 countries were enrolled in the ADVANCE registry, the largest real-world multicenter MCV trial. Women enrolled tended to be slightly older than men (mean 82.2 years vs 79.9 years; P < .001), had higher mean (47.6 vs 43.5 mm Hg; P < .001) and peak (79.0 vs 72.5 mm Hg; P < .001) gradients, and were more symptomatic (83% vs 76% in NYHA class III/IV heart failure; P = .008) [Linke A et al. EuroPCR 2013]. Women also had a lower frequency of coronary artery disease (CAD; 46% vs 70%; P < .001). Clinical end points were reported according to Valve Academic Research Consortium (VARC) criteria. The primary end point was a major adverse cardiac or cerebrovascular event (MACCE) at 30 days post procedure, defined as a composite of all-cause mortality, myocardial infarction (MI), emergent cardiac surgery or percutaneous re-intervention, and stroke. Data showed the effectiveness of the MCV implantation. At 30 days, MACCE had occurred in 9.1% of women vs 7.5% of men (P = .36), and rates of all-cause mortality and cardiovascular mortality were 4.6% vs 4.5% (P = .95) and 3.4% vs 3.5% (P = .90), respectively. Strokes occurred in 4.4% of women vs 1.4% of men (P < .01), and a composite of stroke and transient ischemic attack in 4.6% of women vs 1.8% of men (P = .01). Major vascular complications occurred in 14.1% of women vs 7.1% of men (P < .01), with major bleeding recorded in 12.3% of women vs 7.0% of men (P < .01). In summary, the ADVANCE registry demonstrated that women tended to have similar rates of mortality, but higher rates of stroke/TIA, vascular complications, and bleeding compared with men undergoing TAVR using the MCV prosthesis.
Data from the CoreValve trials subsequently confirmed some of these earlier observations. In the CoreValve US Pivotal Trial to determine the safety and efficacy of self-expanding TAVR at 2 years in patients at extreme risk for surgery, a subgroup analysis determined a numerically, although not significantly, lower rate of all-cause mortality or major stroke in women vs men (25.5% vs 28.6%; P = .1259) at 1 year. At 2 years, these rates had increased (35.1% vs 41.0%; P = .1809) [Yakubov SJ et al. TCT 2014].
The US CoreValve High-Risk Study compared TAVR with surgical aortic valve replacement (SAVR) in 795 patients with severe AS and an increased risk of death during surgery. A subgroup analysis demonstrated a numerically lower all-cause death rate in the TAVR arm in women than men at 1 year (12.7% vs 15.5%) and a numerically higher rate in the SAVR arm (21.8% vs 16.7%; P = .21 for both TAVR and SAVR). At 30 days, bleeding risk was also significantly different between women in the TAVR and SAVR arms (major or life-threatening or disabling bleeding 45.9% vs 74.2%; P < .0001), with similar results at 1 year (48.2% vs 76.7%; P < .0001).
Darshan Doshi, MD, Columbia University Medical Center, New York, New York, USA, discussed the female data from the PARTNER trial [NCT00530894]. This study involved 699 high-risk patients with severe AS who were randomized to undergo either TAVR or SAVR.
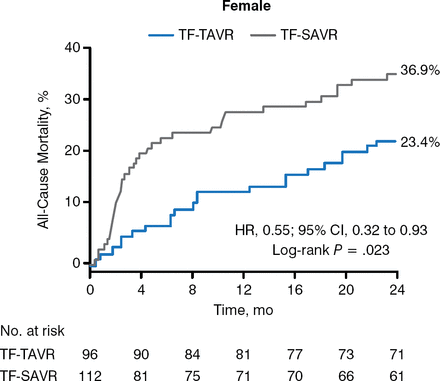
In the operable cohort at 1 year, the primary end point of death from any cause occurred in 24.2% and 26.8% of the 2 groups, respectively (P = .44), and was lower in women who underwent TAVR than SAVR (18.4% vs 27.2%; P = .05) [Smith CR et al. N Engl J Med. 2011], with a 10% absolute risk reduction in women undergoing TAVR compared with men. And although there was no benefit demonstrated for transapical TAVR over transapical SAVR in women (37.3% vs 41.7%), all-cause mortality was significantly reduced with transfemoral TAVR in women (23.4% vs 36.9%; P = .02; Figure 1). Rate of stroke, however, was significantly increased in women in the TAVR compared with the SAVR group, at 30 days (P = .02) and 1 year (P = .007).
All-Cause Mortality in Women Undergoing Transfemoral TAVR
TAVR, transcatheter aortic valve replacement; TF-SAVR, transfemoral surgical aortic valve replacement; TF-TAVR, transfemoral TAVR.
Reproduced with permission from D Doshi, MD.
Dr Doshi also discussed the results from the inoperable arm of the PARTNER trial in which patients were randomized 1:1 to transfemoral TAVR or standard therapy. At 2 years, there was an almost 20% reduction in all-cause mortality in women who underwent TAVR compared with standard therapy (44.4% vs 63.9%; P = .008).
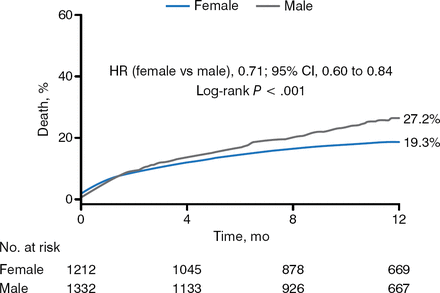
And in an observational analysis of the entire PARTNER study population of patients (n = 2544) with severe AS undergoing TAVR, 30-day outcomes, including all-cause mortality (P = .52) and stroke (P = .26) were similar in women and men. However, women undergoing TAVR had greater major vascular complications (8.5% vs 4.1%; P < .0001) and major bleeding (10.3% vs 7.8%; P = .03) vs men at 30 days. Female sex was one of the most significant predictors of major vascular complications at 30 days (P = .0012). Yet, despite these complications, women still experienced lower 1-year mortality rates than men (19.3% vs 27.2%; P < .001; Figure 2).
1-Year Mortality Rates Following TAVR
TAVR, transcatheter aortic valve replacement.
Reproduced with permission from D Doshi, MD.
Dr Doshi summarized by stating that, in operable patients, there is similar benefit of TAVR over standard therapy for women and men. But in high-risk patients, the increased risk of female sex observed in SAVR risk models of 30-day outcomes does not appear to be present in patients undergoing TAVR.
Alaide Chieffo, MD, San Raffaele Scientific Institute, Milan, Italy, discussed the WIN TAVI registry [NCT01819181]. This aims to determine the safety and efficacy of transcatheter aortic valve implantation (TAVI) in women, according to VARC 2 and Bleeding Academic Research Consortium (BARC) definitions, by collecting specific sex-related issues that are not captured in other registries, in an attempt to address the lack of available sex-based data.
This multicenter, prospective, observational registry has so far recruited 17 study sites in Europe, and aims to enroll at least 1000 female patients with severe AS who are undergoing TAVI with commercially available valves and delivery systems at participating centers.
Dr Chieffo discussed some of the unique female-specific data points demonstrated thus far, including a history of pregnancy (70.19%), hypertension during pregnancy (2.71%), breastfeeding (43.88%), hormone-replacement therapy (6.16%), and osteoporosis (18.87%). The mean age at menopause is 48.6 years.
In her concluding remarks, Dr Chieffo noted that the registry also involves 2 substudies. The Echo substudy aims to collect baseline characteristics of the aortic stenosis and left ventricular function and to obtain follow-up characteristics of the TAVI. The computed tomography (CT) scan substudy aims to assess aortic annulus and vascular access measurements of women undergoing TAVI and to determine the predictive value of the aortic annulus and vascular access measurements with CT for specific complications.
- © 2014 MD Conference Express®