Summary
Results from the Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve Month Dual Antiplatelet Therapy trial [SECURITY; Colombo A et al. J Am Coll Cardiol. 2014. In press] have demonstrated the noninferiority of postprocedural dual antiplatelet therapy for 6 months as compared with 12 months in non-high-risk patients undergoing percutaneous coronary intervention with a second-generation drug eluting stent.
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology
- Cardiology Clinical Trials
- Interventional Techniques & Devices
Results from the Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve Month Dual Antiplatelet Therapy trial [SECURITY; Colombo A et al. J Am Coll Cardiol. 2014. In press] have demonstrated the noninferiority of postprocedural dual antiplatelet therapy (DAPT) for 6 months as compared with 12 months in non-high-risk patients undergoing percutaneous coronary intervention (PCI) with a second-generation drug eluting stent (2G-DES). The trial findings were described by Antonio Colombo, MD, Centro Cuore Columbus, Milan, Italy.
The SECURITY trial was a prospective, randomized, non-inferiority, investigator-driven trial. The main inclusion criteria were the presence of coronary artery disease with stable angina, unstable angina or asymptomatic documented ischemia as clinical manifestations. Additional inclusion criteria were patient age over 18 years, no other DES implanted before the target procedure, and no bare metal stent implanted in the 3 months before the target procedure. The main exclusion criteria were history of treatment for venous or arterial graft lesions, in-stent restenosis, unprotected left main lesions, STEMI within 48 hours of surgery, non-STEMI in the prior 6 months. Additional exclusion criteria were left ventricular ejection fraction (LVEF) ≤ 30%; hypersensitivity or allergy to study drug or devices; chronic renal insufficiency; diagnosed life expectancy < 24 months; and current participation in another study involving a drug or device.
The primary end point was a composite of cardiac death, MI, stroke, stent thrombosis, or Bleeding Academic Consortium Criteria (BARC) type 3 or 5 bleeding at 12 months. Conversely, secondary end points included the composite of cardiac death, MI, stroke, definite/probable stent thrombosis or BARC type 2, 3, or 5 bleeding at 12 and 24 months, and the occurrence of MI, ischemia-driven revascularization (with PCI or coronary artery by-pass graft), all bleeding events, and all-cause mortality at 30 days and 6, 12, and 24 months.
The analysis was carried out according to the intention to treat principle. Additionally, it was prespecified as a per-protocol analysis including only patients fulfilling all major inclusion criteria and treated according to the assigned randomization arm.
Overall, 1399 patients were randomized to the 6-month (n = 682) or 12-month (n = 717) DAPT regimen. The randomization arms were well balanced in terms of baseline clinical and angiographic characteristics. Use of medication during the trial was similar at 6 months. Conversely, more than 30% of patients in the 6-month group were on DAPT regimen at 12 months.
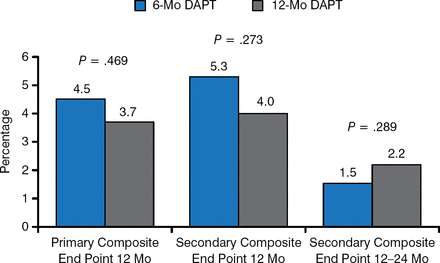
There were no statistically significant differences between the 6- and 12-month regimens concerning the primary and secondary composite end points at 12 months (Figure 1).
Primary and Secondary Composite End Points
DAPT, dual antiplatelet therapy.
Reproduced with permission from A Columbo, MD.
Moreover, no differences in the secondary end points incidence at 24 months were observed between the study groups.
Rates of the single end points are reported in Table 1. Again, no differences were observed across the different outcomes between 6- and 12-month regimens. Of note, only 6 cases of definite/probable stent thrombosis occurred at follow-up.
Secondary End Points
Multivariable analysis revealed a significant association between the primary end point and age, type of stent implanted and stent number/length/size (Table 2). Interestingly, diabetes mellitus was only of borderline statistical significance in this trial.
Predictors of the Primary End Point
Despite several study limitations, such as the lower than expected primary end point incidence and statistical power, Dr Colombo concluded that the 6-month DAPT is noninferior to the 12-month regimen in low-risk patients undergoing PCI with a 2G-DES.
- © 2014 MD Conference Express®