Summary
In the treatment of plantar fasciitis, micronized amniotic membrane (c-hAM) injection demonstrated similar efficacy to corticosteroid injection without adverse events. This article presents data from a randomized, controlled, double-blinded, single-center, prospective study comparing plantar fasciitis injection of c-hAM to steroid injection.
- Foot & Ankle Conditions
- Orthopaedics Clinical Trials
- Foot & Ankle Conditions
- Orthopaedics
- Orthopaedics Clinical Trials
In the treatment of plantar fasciitis, micronized amniotic membrane (c-hAM) injection demonstrated similar efficacy to corticosteroid injection without adverse events. Robert D. Santrock, MD, West Virginia University, Morgantown, West Virginia, USA, presented data from a randomized, controlled, double-blinded, single-center, prospective study comparing plantar fasciitis injection of c-hAM to steroid injection [Hanselman AE et al. Foot Ankle Int. 2014].
Fetal tissues, including the amnion, are currently used in ophthalmology, orthopedics, and other surgical specialties. Use of fetal tissues in surgery is appealing because the tissue is able to regenerate without inflammation or scarring. The study hypothesis was that c-hAM is a safe treatment option for plantar fasciitis and is noninferior to corticosteroids.
In this study, 23 patients diagnosed with plantar fasciitis and symptomatic for > 3 months but < 1 year were randomly assigned to receive an initial injection of either 1 mL (40 mg) of corticosteroid plus 4 mL 0.5% bupivacaine, or 1 mL c-hAM plus 4 mL 0.5% bupivacaine. At 6-week follow-up, all patients were given the option to receive a repeat injection of either study drug if needed. Patients were followed for 12 weeks after the most recent injection. Activities were not restricted, but all patients were advised to perform foot and ankle-stretching exercises 5 times a day.
Exclusion criteria included previous plantar fasciitis injection or treatment within 3 months, previous foot surgery or injury, lower extremity neuropathy, lack of ambulation, or unwillingness to receive human tissue. The primary end point was the Foot Health Status Questionnaire (FHSQ). Secondary end points included the visual analog scale (VAS) and patient-reported improvement.
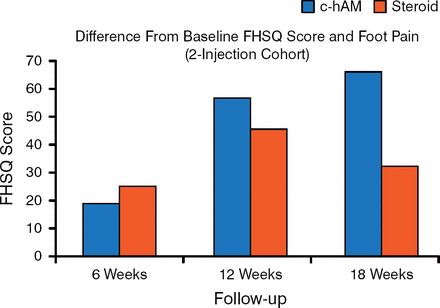
There was no significant difference between the 2 arms in terms of foot pain, foot function, and VAS. However, in the 1-injection cohort, patients who received the corticosteroid demonstrated greater FHSQ foot pain scores at 6 and 12 weeks compared with patients who received c-hAM. Interestingly, in the 2-injection cohort, patients who received c-hAM demonstrated a trend toward a greater FHSQ foot pain score compared with the corticosteroid arm (Figure 1). A similar trend was observed in FHSQ foot function, in which 2 injections of c-hAM demonstrated increased effectiveness compared with 1 injection. After 2 injections, the c-hAM arm trended toward a greater improvement in VAS and patient-reported improvements at 18 weeks compared with the corticosteroid arm. There were no adverse events in this study.
Effect of 2 Injections of c-hAM on FHSQ Score
c-hAM, micronized amniotic membrane; FHSQ, Foot Health Status Questionnaire.
Reproduced with permission from R Santrock, MD.
According to Dr Santrock, the data from this study suggest that c-hAM has comparable efficacy to corticosteroids in the treatment of plantar fasciitis, and there may be a double-dose effect associated with c-hAM. He indicated that that a larger, longer-term, multicenter trial is needed to further evaluate c-hAM for plantar fasciitis.
- © 2014 MD Conference Express®