Summary
Grazoprevir is a highly potent hepatitis C virus (HCV)-specific NS3/4A protease inhibitor. Part A of a Study of the Combination Regimen Grazoprevir (MK-5172) and Elbasvir (MK-8742) ± Ribavirin in Participants With Chronic Hepatitis C [C-WORTHy; NCT01717326] reported efficacy of 89% to 100% in treatment-naïve noncirrhotic patients with HCV genotype-1 infection. This article discusses the expanded C-WORTHy Part B, which assessed the efficacy and safety of grazoprevir plus elbasvir with or without ribavirin in patients with HCV monoinfection and patients with HCV and human immunodeficiency virus coinfection.
- Hepatology Clinical Trials
- Liver Conditions
- Viral Infections
- Hepatology
- Hepatology Clinical Trials
- Liver Conditions
- Viral Infections
Grazoprevir (MK-5172) is a highly potent hepatitis C virus (HCV)-specific NS3/4A protease inhibitor. Elbasvir (MK-8742) is a highly potent HCV-specific NS5A inhibitor. The all-oral combination of grazoprevir and elbasvir provides a high barrier to HCV resistance. Part A of a Study of the Combination Regimen Grazoprevir (MK-5172) and Elbasvir (MK-8742) ± Ribavirin in Participants With Chronic Hepatitis C (MK-5172-035) (C-WORTHy) [NCT01717326] reported efficacy of 89% to 100% in treatment-naïve noncirrhotic patients with HCV genotype (GT)-1 infection [Lawitz E et al. Lancet. 2014], supporting expansion in Part B to more diverse populations. The aim of C-WORTHy Part B, presented by Mark S. Sulkowski, MD, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA, was to assess the efficacy and safety of grazoprevir plus elbasvir with or without ribavirin in patients with HCV monoinfection and patients with HCV and human immunodeficiency virus (HIV) coinfection.
Included patients had HCV GT-1, and they were noncirrhotic and HCV treatment-naïve. Coinfected patients were stable on raltegravir plus 2 nucleoside reverse transcriptase inhibitors (NRTIs) for ≥ 2 weeks before enrollment, had CD4 counts > 300 cells/mm3, had undetectable HIV RNA for 24 weeks, and had not failed > 1 prior anti-HIV regimen. A total of 159 HCV monoinfected patients and 59 HCV and HIV coinfected patients were enrolled. The patients were stratified by HCV sub-GT. In the monoinfected group, patients with GT-1a received grazoprevir plus elbasvir 50 mg plus ribavirin for 8 weeks. Monoinfected patients with GT-1a and 1b received grazoprevir plus elbasvir (20 or 50 mg) plus ribavirin (n = 85) or grazoprevir plus elbasvir 50 mg without ribavirin. In the coinfected group, patients with HCV GT-1a and 1b received grazoprevir plus elbasvir 50 mg with ribavirin (n = 29) or without ribavirin (n = 30). Patients with GT-1a and 1b were treated for 12 weeks. The end points were sustained viral response at 12 weeks post treatment (sustained virologic response at week 12; SVR12) and at 24 weeks post treatment (SVR24).
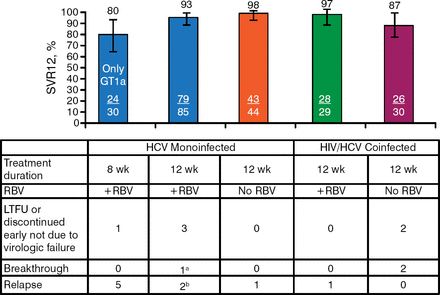
The primary end point of SVR12 in the HCV monoinfected patients was achieved by 80% of the GT-1a group, 93% of the GT-1a and 1b ribavirin-treated group, and 98% of the GT-1a and 1b no-ribavirin group. Among coinfected GT-1a and 1b patients, 97% of ribavirin-treated patients and 87% of no-ribavirin patients achieved SVR12 (Figure 1).
Sustained Virologic Response at Week 12 Results: Intention to Treat
GT, genotype; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LTFU, lost to follow-up; RBV, ribavirin; SVR12, sustained viral response at 12 wk post treatment.
aBreakthrough was due to HCV GT-2b (minor GT-2b variant at baseline).
bOne of the patients who relapsed did not receive grazoprevir and only received elbasvir + RBV for the first month of treatment.
Reproduced with permission from MS Sulkowski, MD.
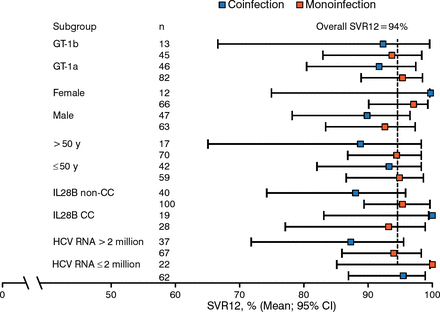
Among all patients treated for 12 weeks, the SVR12 rate in ribavirin-treated patients was 95% in those with GT-1a and 92% in those with GT-1b. The SVR12 rate in no-ribavirin patients was 92% in those with GT-1a and 95% in those with GT-1b. Figure 2 shows a subgroup analysis of SVR12 rates, and Table 1 shows virologic failure rates.
Subgroup Analysis of SVR12 Rates (12 Week Arms Only)
CC, having 2 C alleles; GT, genotype; HCV, hepatitis C virus; IL28B, interleukin 28B; SVR12, sustained viral response at 12 wk post treatment.
Reproduced with permission from MS Sulkowski, MD.
Virologic Failure Rates 4% (7/188) of patients treated for 12 wk 17% (5 relapses/30) of patients treated for 8 wk
The safety profile was similar in monoinfected and coinfected patients, with no discontinuations due to adverse events or laboratory abnormalities. The most common adverse events were fatigue, headache, nausea, and diarrhea.
Treatment with grazoprevir plus elbasvir with or without ribavirin for 12 weeks produced high efficacy rates in patients with HCV monoinfection and patients with HCV and HIV coinfection. Grazoprevir plus elbasvir with or without ribavirin was generally safe and well tolerated in both patient groups. All coinfected patients suppressed HIV and had stable CD4 counts. These results support the ongoing phase 3 development of grazoprevir plus elbasvir.
- © 2014 MD Conference Express®