Summary
Severe alcoholic hepatitis, has a 28-day mortality rate of about 35%. Clinical trials have shown both corticosteroids and pentoxifylline (PTX) to be of potential therapeutic benefit, however, both are controversial. This article discusses data from the Steroids or Pentoxifylline for Alcoholic Hepatitis trial [STOPAH; ISRCTN88782125].
- Hepatology Clinical Trials Liver Conditions
- Hepatology Clinical Trials
- Hepatology
- Liver Conditions
Severe alcoholic hepatitis, defined by a Maddrey's discriminant function (DF) ≥ 32, has a 28-day mortality rate of about 35%. Clinical trials have shown both corticosteroids and pentoxifylline (PTX) to be of potential therapeutic benefit, and both are recommended in current practice guidelines [Malthurin P et al. J Hepatol. 2012; O'Shea RS et al. Hepatology. 2010]. However, both are controversial, steroids because of inconsistent trial outcomes and PTX because its use is based on a single trial. Mark Richard Thursz, MD, Imperial College London, London, UK, presented data from the Steroids or Pentoxifylline for Alcoholic Hepatitis trial [STOPAH; ISRCTN88782125], which confirmed a mortality benefit with prednisolone at 28 days. PTX had no impact on disease progression.
STOPAH was a randomized, double-blind, placebo-controlled phase 3 trial designed to assess the efficacy of prednisolone 40 mg daily or PTX 400 mg 3 times daily in the treatment of severe alcoholic hepatitis [Forrest E et al. Trials. 2013]. The primary end point was mortality at 28 days. Secondary end points included mortality/transplant at 90 days and 12 months, and diagnostic utility of existing prognostic scores. Other objectives were to assess rates of recidivism and the impact of recidivism on subsequent survival. The study included patients aged ≥ 18 years with a clinical diagnosis of severe alcoholic hepatitis (ie, DF ≥ 32), a serum bilirubin > 80 μmol/L, and a history of excess alcohol consumption (> 80 g/d for men; > 60 g/d for women) who had been hospitalized for < 4 weeks. Abstinence of > 6 weeks prior to randomization, jaundice lasting > 3 months, and/or use of either study drug within 6 months were causes for exclusion. Participants were randomized to 1 of 4 groups—placebo/placebo, prednisolone/placebo, placebo/PTX, or prednisolone/PTX—and treated for 4 weeks.
Subjects (n = 1092) were mean age 48.7 years (62.7% men; 96% white) with a mean alcohol consumption of 200.1 g/d (149.5 g/day for women). Mean time from admission to treatment was 6.4 days. About 27% exhibited encephalopathy on admission. Laboratory values were similar to those seen in other trials.
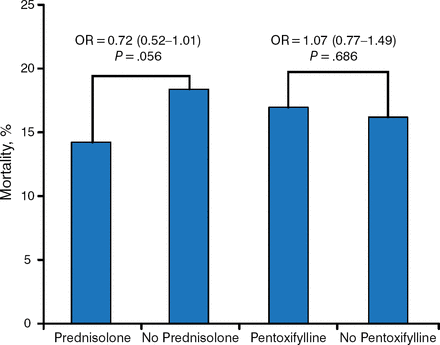
The overall mortality rate was 16%. The mortality rate for subjects treated with prednisolone was 13.9% vs 18% for subjects not receiving prednisolone. Mortality among subjects treated with PTX was 16.4% vs 15.5% for those not receiving PTX (Table 1).
Mortality at 28 Days, % (n/N)
The odds ratio for 28-day mortality was 0.72 (95% CI, 0.52 to 1.01; P = .056) for prednisolone compared with 1.07 (95% CI, 0.77 to 1.49; P = .686) for PTX (Figure 1).
28-Day Mortality
Reproduced with permission from MR Thursz, MD.
On multivariate analysis, accounting for disease severity and prognosis, the 28-day odds ratio for mortality in the prednisolone group was 0.609 (95% CI, 0.409 to 0.900; P = .015), indicating a potential reduction in mortality of about 40%. Neither treatment had any impact on survival beyond 28 days. Infection, in particular lung infection, was more common in prednisolone-treated subjects.
All 4 prognostic scoring systems (ie, DF, Glasgow Alcoholic Hepatitis Score [GAHS], Model for End Stage Liver Disease [MELD], and the Lille model) were significantly associated with prognosis (all P < .0001). However, the area under the receiver operating characteristic curves (AUROC) were relatively low ranging from .69 to .74.
Abstinence is a major determinant of survival after 90 days. Compared with patients who reported complete abstinence, the 90-day odds ratio for mortality for those who reduced their drinking to within government guidelines was double. For those who did not reduce their drinking, it was triple (Table 2).
Abstinence and Mortality
- © 2014 MD Conference Express®