Summary
Benign hepatocellular adenomas are not a uniform liver disease but include different types of tumors, which can be classified as hemangioma (nonepithelial), focal nodular hyperplasia, and hepatocellular adenoma, the latter 2 being epithelial tumors. This article discusses how insights from molecular biology have profoundly changed our clinical understanding of benign liver tumors and how using the genotype-phenotype classification of these tumors can identify new etiologies, as well as diagnostic and prognostic features of this disease.
- Liver Conditions
- Hepatology
- Liver Conditions
Benign hepatocellular adenomas are not a uniform liver disease but include different types of tumors, which can be classified as hemangioma (nonepithelial), focal nodular hyperplasia (FNH), and hepatocellular adenoma (HCA), the latter 2 being epithelial tumors. Prof Jessica Zucman-Rossi, Université Paris Descartes, Paris, France, discussed how insights from molecular biology have profoundly changed our clinical understanding of benign liver tumors and how using the genotype-phenotype classification of these tumors can identify new etiologies, as well as diagnostic and prognostic features of this disease.
Of the two main hepatocellular tumor types, 90% are classified as FNH and result in no complications. The remaining 10% of cases are HCAs, which are associated with bleeding (27%) and transformation to hepatocellular carcinoma (HCC, 4.2%). Patient management is based on 3 questions: (1) determine whether the patient has FNH, HCA, or HCC; (2) identify and prevent risk factors; and (3) evaluate the risk of malignant transformation and hemorrhages.
Until recently, it has been difficult to differentiate these tumors, but the advent of molecular classification of benign liver tumors has made it possible to better define the lesions and their molecular subtypes and to identify new biomarkers that can be used to better assess their pathology. FNH is more common in females (F:M = 8:1). Oral contraceptives play only a limited role in the development of FNH, and there is usually no bleeding or malignant transformation. FNH is defined by the accumulation of mature normal hepatocytes that are arranged in plates 1 to 2 cells thick and centered on a fibrous central scar with dystrophic arterial vessels that are located in the center of the lesion. There are also ductular reactions at the interface of the fibrous scar. FNH is a type of hyperplastic lesion composed of a reactive proliferation of hepatocytes that is due to a polyclonal proliferation of hepatocytes. The occurrence of FNH is related to a preexisting vascular malformation associated with an increased expression of angiopoietin 1 (proteins involved in angiogenesis) but also to an overexpression of transforming growth factor beta (TGFβ) and platelet-derived growth factor (PDGF) at the central scar. WNT/β-catenin is also activated and is involved in the benign hepatocyte proliferation and metabolic zonation in the liver. A typical maplike pattern of glutamine synthetase (GS) expression shows an altered zonation in FNH, and it is a good marker for easily identifying resected FNH from other hepatocellular nodules [Bioulac-Sage P et al. Liver Int. 2008; Rebouissou S et al. J Hepatol. 2008].
HCAs are rare benign monoclonal liver tumors mainly observed in young women after oral contraceptive use. They may be seen as solitary adenomas, multiple (2 to 4 nodules), or adenomatosis (> 10 nodules). Molecular subtypes include mutation-inactivating hepatocyte nuclear factor 1 alpha (HNF1A; 30% to 40% of tumors), inflammatory (40% to 50%), β-catenin activation (12% to 19%), and unclassified with nonspecific features (10%).
HNF1A tumors are phenotypically characterized by marked homogeneous steatosis. Bi-allelic inactivation and heterozygous germline mutations of HNF1A are associated with familial hepatic adenomatosis and maturity-onset diabetes of the young (MODY) type 3 [Reznik Y et al. J Clin Endocrinol Metab. 2004]. Patients with liver adenomatosis should be tested for familial diabetes and HNF1A germline mutations.
The subgroup of tumors classified as inflammatory lesions are characterized by the presence of inflammatory infiltrates, sinusoidal dilatation, and dystrophic vessels [Zucman-Rossi J et al. Hepatology. 2006]. Inflammatory adenomas are defined by a STAT3 activation explained in 80% of the cases by activating mutations in either gp130, STAT3, FRK, JAK1, or GNAS genes. Rare unexplained inflammatory syndromes can be caused by inflammatory adenoma and cured by tumor resection. Risk factors of inflammatory-type tumors include obesity and excessive alcohol use. Glycogen storage disease is a rare hereditary metabolic disease resulting from deficiency of the glucose-6-phosphate complex, which can predispose an individual to inflammatory hepatocellular adenomas [Calderaro J et al. J Hepatol. 2013]. A positive serum amyloid protein is the immunohistochemistry (IHC) marker for inflammatory adenomas.
Tumors defined by β-catenin activation have frequent cytological abnormalities, pseudoglandular formation, and cholestasis. CTNNB1 (cadherin-associated protein) encodes β-catenin, and somatic mutations at exon 3 result in high β-catenin activity, whereas those at exon 7 and exon 8 result in low β-catenin levels. GS and positive β-catenin are IHC markers for inflammatory lesions. A high percentage (38%) of this subtype occurs in males. Androgen therapy and anabolic intake are risk factors for the development of β-catenin adenomas. Although androgens should be stopped when possible, there is a risk of malignant transformation even after stoppage.
Prior to the identification of these different subtypes, imaging features of HCAs were nonspecific. Now, it is well established that the different molecular features correspond to distinct phenotypical features that can be validated by magnetic resonance imaging (MRI). For instance, diffuse signal dropout on T1-weighted chemical shift sequence is a specific MRI feature of HNF1A-inactivated adenoma.
As mentioned, hemorrhage at diagnosis is associated with HCA in about 27% of cases and is more frequent in large (> 5 cm) HCA adenomas [van Aalten SM et al. Br J Surg. 2012]. Malignant transformation of HCAs into hepatocellular carcinomas, although rare, occurs in 4.2% of cases, almost all in tumors exceeding 5 cm in size [Stoot JH et al. HPB (Oxford). 2010]. High activation of the WNT/β-catenin pathway (through the CTNNB1 exon 3 mutation), the earliest event, is associated with a high risk of malignant transformation, whereas low activity of WNT/β-catenin signaling (through CTNNB1 exon 7 and 8 mutations) is associated with no increased risk of transformation. Exome sequencing identified mutations that induce constitutive kinase activity, STAT3 activation, and telomerase activation by telomerase reverse transcriptase promoter [Pilati C et al. Cancer Cell. 2014]. Telomerase activation was seen as crucial for malignancy.
Future developments in this area might include a better understanding of the role of estrogen exposure in HCA development, as well as more clarity regarding the mechanism of the occurrence of multiple adenomas. New markers to predict hemorrhages and an evaluation of molecular classification in routine clinical care are needed.
HCA molecular classification can help in diagnoses at imaging and histological levels.
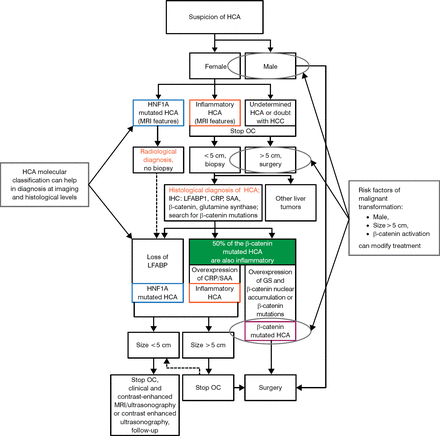
Knowing the risk factors of malignant transformation (male, size > 5 cm, β-catenin activation) can modify treatment. A complicated but specific composite algorithm for the diagnosis and treatment of HCA has been proposed by Nault JC et al. [Figure 1; Gastroenterology. 2013]
Algorithm for the Diagnoses and Treatment of Hepatocellular Adenoma
CRP, C-reactive protein; GS, glutamine synthetase; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; HNF1A, hepatocyte nuclear factor 1 alpha; IHC, immunohistochemistry; LFABP, liver-type fatty acid-binding protein; MRI, magnetic resonance imaging; OC, oral contraception; SAA, serum amyloid A.
Adapted with permission of AGA Institute, from Nault JC et al. Hepatocellular Benign Tumors—From Molecular Classification to Personalized Clinical Care. Gastroenterology. 2013;144:888–902. Copyright © 2013. Permission conveyed through Copyright Clearance Center, Inc.
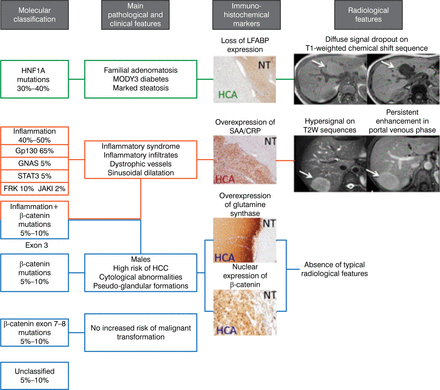
Dr Zucman-Rossi concluded by saying that “molecular biology has profoundly changed our knowledge of the benign liver tumors.” Using the genotype-phenotype HCA classification, as shown in Figure 2 [Nault JC et al. Gastroenterology. 2013], can identify new etiologies and diagnostic and prognostic features of the disease, leading to more personalized medicine.
Using the Genotype-Phenotype Hepatocellular Adenoma Classification for Personalized Treatment
CRP, C-reactive protein; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; HNF1A, hepatocyte nuclear factor 1 alpha; LFABP, liver-type fatty acid-binding protein 1; MODY3, maturity-onset diabetes of the young type 3; NT, normal tissue; SAA, serum amyloid A; T2W, T2-weighted.
Adapted with permission of AGA Institute, from Nault JC et al. Hepatocellular Benign Tumors—From Molecular Classification to Personalized Clinical Care. Gastroenterology. 2013;144:888–113; 902. Copyright © 2013. Permission conveyed through Copyright Clearance Center, Inc.
- © 2014 MD Conference Express®