Summary
This article discusses new research on atrial fibrillation (AF) and how it may contribute to disease management, the use of novel oral anticoagulants in periprocedural anticoagulation management, the clinical overlap between AF and heart failure, as well as an overview of new ACC/AHA/HRS guideline recommendations.
- arrhythmias
- cardiology guidelines
- heart failure
Stanley Nattel, MD, Montreal Heart Institute, Montreal, Quebec, Canada, reviewed new research on atrial fibrillation (AF) and how it may contribute to disease management. He focused on 3 lessons: First, using the reentrant rotor concept is necessary and applicable to understand important aspects of AF; second, targeting dormant conduction can prevent AF recurrence; and, third, AF substrate progression owing to underlying risk factors is preventable.
NEW RESEARCH ON AF
Although the standard wavelength theory of reentry suggests that Na+ channel blockers should make AF more persistent, recent data indicate that Na+ channel inhibition can terminate AF through its effect on reentrant rotors [Kneller J et al. Circ Res. 2005]. This concept is supported by the CONFIRM trial [Narayan SM et al. J Am Coll Cardiol. 2012], which showed that localized electrical rotors and focal impulse sources are prevalent, sustaining mechanisms for AF and that ablation at patient-specific sources acutely terminated or slowed AF and improved the outcome.
AF recurs in ≤ 50% of patients after catheter ablation, usually as a result of recovery from pulmonary vein conduction. In the ADVICE randomized trial [Macle L et al. HRS 2014. (abstr LB01-02)], the use of intravenous adenosine elicited dormant conduction in > 50% of patients. After a mean follow-up of 1 year, 69% of these patients who received a single additional adenosine-guided ablation were free of AF, compared with 42% of those who received no additional ablation (P < .0001).
NOACs IN PERIPROCEDURAL ANTICOAGULATION MANAGEMENT
In deciding whether it is necessary to interrupt anticoagulation therapy for procedures including ablation, the following aspects of procedures must be considered: bleeding risk (Table 1), duration of interrupted therapy (if there is an interruption), and whether bridging is necessary. Elaine Hylek, MD, MPH, Boston University Medical Center, Boston, Massachusetts, USA, discussed the use of novel oral anticoagulants (NOACs) in periprocedural anticoagulation management.
Procedure-Related Bleeding Risk
Dr Hylek noted that several studies have shown similar periprocedural bleeding with vitamin K antagonists and NOACs, including RE-LY [Healey JS et al. Circulation. 2012], ROCKET AF [Sherwood MW et al. Circulation. 2014], and ARISTOTLE [Garcia D et al. Blood. 2014].
With respect to the need for bridging, she suggested that the short time to maximum plasma concentration with NOACs versus warfarin obviates the need for bridging but highlights the importance of hemostasis before resumption of treatment. For urgent reversal of vitamin K antagonist therapy in major bleeding events, Dr Hylek believes that there is good evidence for the use of a 4-factor prothrombin complex concentrate as an effective alternative to plasma [Sarode R et al. Circulation. 2013].
As for their use in cardioversion, Dr Hylek concluded that the data to date suggest that NOACs are efficacious and safe [Flaker G et al. J Am Coll Cardiol. 2014; Nagarakanti R et al. Circulation. 2011]. Future studies are planned for cardioversion and catheter ablation.
RELATIONSHIP BETWEEN AF AND HF
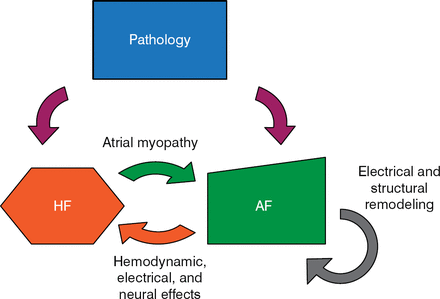
There is an important mechanistic and clinical overlap between AF and heart failure (HF; Figure 1). Clyde W. Yancy Jr, MD, Northwestern University, Chicago, Illinois, USA, discussed what is known about this relationship.
The Overlapping Pathology of AF and HF
AF, atrial fibrillation; HF, heart failure.
Reproduced with permission from CW Yancy, Jr, MD.
The prevalence of AF in patients enrolled in HF studies varies from a low of < 10% to a high of 50% [Trulock KM et al. J Am Coll Cardiol. 2014]. The 2 conditions share many of the same risk factors, including coronary disease, hypertension, tobacco use, obesity, diabetes, kidney disease, and sleep apnea. The presence of both AF and HF is associated with a worse prognosis than that for either condition alone [Wang TJ et al. Circulation. 2003].
The current treatment guidelines for AF emphasize rate control and anticoagulation [January CT et al. Circulation. 2014]. However, quality improvement data in the IMPROVE HF study [Fonarow GC et al. Circ Heart Fail. 2008] show that the use of guideline-recommended therapies for patients with AF and HF vary widely. In particular, the use of anticoagulation therapy varies in clinical practice, especially among older patients [Hernandez AF et al. Circ Cardiovasc Qual Outcomes. 2014].
The benefits of rhythm control in patients with both AF and HF remain uncertain [Trulock KM et al. J Am Coll Cardiol. 2014]. In the AFFIRM study [Wyse DG et al. N Engl J Med. 2002], there was a potential advantage with rhythm control among patients with AF and HF, while in the AF-CHF study [Talajic M et al. J Am Coll Cardiol. 2010], no differences were noted for a rate-versus-rhythm approach on the primary or secondary end points. More recently, in a meta-analysis of 26 studies in patients (n = 1838) with left ventricular systolic dysfunction undergoing catheter ablation for AF, left ventricular ejection fraction improved significantly during follow-up by 13% (P < .001) [Anselmino M et al. Circ Arrhythm Electrophysiol. 2014]. Ongoing studies, such as RAFT-AF [NCT01420393] and CASTLE-AF [NCT00643188], may provide important answers concerning the benefit of a rhythm strategy in these patients.
Both obesity and obstructive sleep apnea are additional risk factors for AF but are reversible. Recent studies have shown that weight reduction combined with intensive management of cardiometabolic risk factors reduces AF symptom burden and severity [Abed HS et al. JAMA. 2013], while treatment with continuous positive airway pressure in patients with obstructive sleep apnea is associated with a lower recurrence of AF [Naruse Y et al. Heart Rhythm. 2013].
OAC THERAPY IN PATIENTS WITH AF
Jeff S. Healey, McMaster University, Hamilton, Ontario, Canada, noted that the benefit of oral anticoagulants (OACs) in the AF population is unclear and depends on several factors, including the burden of the AF (singular or ongoing episodes), the presence of other stroke risk factors, and the individual risk/benefit of OAC therapy.
Among elderly individuals with AF, short subclinical atrial fibrillation (SCAF) episodes are frequently detected on pacemaker readouts. Despite the absence of clinical symptoms and their short duration, according to the ASSERT study [Healey JS et al. N Engl J Med. 2012], these episodes are associated with an increased risk of stroke and systemic embolism (Table 2). There is a suggestion that embolism risk is greater in patients with longer episodes of SCAF; however, the small number of events in ASSERT precludes definitive conclusions.
ASSERT Outcomes Based on Presence or Absence of Device-Detected Atrial Tachyarrhythmia
The ARTESIA trial [NCT01938248] is a phase 4 study designed to examine whether treatment with apixaban, compared with aspirin, will reduce the risk of ischemic stroke and systemic embolism in patients with device-detected SCAF and additional risk factors for stroke. The study is not yet open for enrollment but expects to recruit 4000 adults with (1) a permanent pacemaker or defibrillator or insertable cardiac monitor capable of detecting SCAF, (2) at least 1 episode of SCAF ≥ 6 minutes but no single episode > 24 hours, and (3) a CHA2DS2-VASc score ≥ 4.
Pending the outcome of this study, Dr Healey suggested that it seems reasonable to offer OACs to patients with long episodes (> 24 hours) or those with high stroke risk (ie, recent cryptogenic stroke) regardless of SCAF duration.
NEW ACC/AHA/HRS GUIDELINE RECOMMENDATIONS
Finally, Craig T. January, MD, PhD, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA, discussed some of the new recommendations from the 2014 American College of Cardiology (ACC)/American Heart Association (AHA)/ Heart Rhythm Society (HRS) Guideline for the Management of Patients With Atrial Fibrillation [January CT et al. Circulation. 2014], which were developed in collaboration with the society of thoracic surgeons.
The new ACC/AHA/HRS recommendations reflect a paradigm shift from identifying patients at high risk for thromboembolism in need of long-term anticoagulation to identifying low-risk patients not requiring long-term anticoagulation. New class I stroke/thromboembolism recommendations encourage individualized therapy based on shared decision making (level of evidence [LOE] C), the selection of antithrombotic therapy based on the risk of thromboembolism irrespective of the AF pattern (LOE B), and use of the CHA2DS2-VASc score (vs CHADS2) to assess stroke risk in patients with nonvalvular AF (LOE B).
There are also new class I anticoagulation recommendations. For patients with nonvalvular AF with a CHA2DS2-VASc score ≥ 2, the recommended OACs include warfarin (international normalized ratio, 2.0 to 3.0; LOE A) as well as dabigatran, rivaroxaban, and apixaban (all LOE B). Dabigatran, rivaroxaban, and apixaban are also recommended for patients with nonvalvular AF who are unable to maintain a therapeutic international normalized ratio level with warfarin (LOE C). In patients with atrial flutter, antithrombotic therapy should be managed via the same risk profile used for AF (LOE C).
New class I recommendations for the use of rate control in patients with AF include use of a β-blocker or non-dihydropyridine calcium channel antagonist for control of ventricular rate for patients with paroxysmal, persistent, or permanent AF (LOE B). Electrical cardioversion is now indicated in hemodynamically unstable patients (LOE B). Lenient rate control (resting heart rate < 110 beats per minute) may be reasonable when patients remain asymptomatic and left ventricular systolic function is preserved.
Key recommendations for rhythm control now state that in the case of AF ≥ 48 hours or unknown duration, patients should receive anticoagulation with warfarin (INR 2.0 to 3.0; Class I, LOE B). Class I, LOE C recommendations after cardioversion include anticoagulation for ≥ 4 weeks for AF lasting > 48 hours; intravenous heparin or LMWH or a factor Xa or direct thrombin inhibitor for AF 48 hours and a high stroke risk; and long-term anticoagulation based on the thromboembolic risk. Pharmacologic conversion with flecainide, dofetilide, propafenone, and intravenous ibutilide is useful for cardioversion of AF or atrial flutter (LOE A); however, electrical cardioversion has greater initial success.
- © 2014 MD Conference Express®