Summary
This session addressed innovative imaging methods used for the evaluation and treatment of atrial fibrillation, with a focus on various echocardiography techniques and cardiac magnetic resonance imaging. Improvements in imaging methods have resulted in higher quality atrial imaging, lower radiation exposure, and improved prognosis of outcomes following AF therapy.
- echocardiography
- atrial fibrosis
- left atrial strain
- angiography
- electroanatomical mapping
- DECAAF
Left atrial (LA) pressure and volume overload lead to LA remodeling, with changes in size, structure, and function. These changes are associated with adverse cardiovascular (CV) outcomes. Assessment of LA size provides important prognostic information regarding the risk of atrial fibrillation (AF), heart failure, stroke, and death, and outcomes of AF cardioversion and ablation. An enlarged left atrium impacts the success of rhythm control and predisposes the patient to recurrence of AF [Camm AJ et al. Eur Heart J. 2010]. Monica Rosca, MD, Prof Dr CC Iliescu Cardiovascular Diseases Institute, Bucharest, Romania, discussed imaging evaluation of the left atrium in patients with AF.
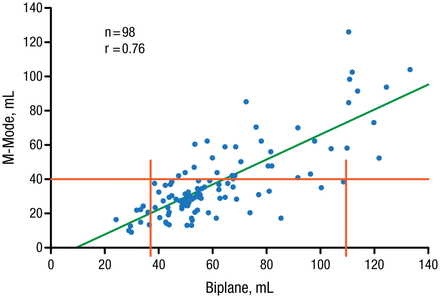
LA enlargement occurs in the superior-inferior or medial-lateral axis. Therefore, M-mode linear dimensions (anteroposterior LA diameter) are not recommended for quantification of LA size (Figure 1) [Evangelista A et al. Eur J Echocardiogr. 2008; Lester SJ et al. Am J Cardiol. 1999].
M-Mode Linear Dimensions Are Inaccurate for Left Atrial Size Assessment
Lester SJ et al. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84:829–832. Copyright (1999), with permission from Excerpta Medica Inc.
LA volume is a more accurate description of the size and is more strongly associated with the risk of CV events compared to LA area or diameter [Tsang TS et al. J Am Coll Cardiol. 2006]. The recommended method for assessment of LA size by echocardiography is the biplane area-length formula, which has been used in most studies [Lang RM et al. J Am Soc Echocardiogr. 2005]. However, the assumptions used may be inaccurate and LA volume is underestimated with this method compared with computed tomography (CT) and magnetic resonance imaging (MRI).
The use of 3D echocardiography (3DE) avoids geometric assumptions and atrial cavity foreshortening, providing higher volumes than 2D echocardiography (2DE). 3DE has been validated against MRI [Rodevan O et al. Int J Card Imaging. 1999] and has been shown to be accurate, with low test-retest variation and lower intraobserver and interobserver variability compared with 2DE [Jenkins C et al. J Am Soc Echocardiogr. 2005]. However, 3DE is limited by a dependence on 2D image quality and lack of cutoffs. The former also provides high-quality images of LA anatomy. Furthermore, 3D transesophageal echocardiography improves left atrial appendage morphology and size assessment, which is important for guiding percutaneous closure [Nucifora G et al. Circ Cardiovasc Imaging. 2011].
Analysis of transmitral or pulmonary venous flow patterns with pulse wave Doppler echocardiography is a simple method for assessing LA function. Pulse wave Doppler echocardiography can be used to identify changes in flow patterns consistent with atrial stunning as well as recovery of atrial mechanical function after restoration of sinus rhythm.
Longitudinal myocardial velocities can be quantified with tissue Doppler imaging, providing a relatively load-independent measure of left ventricular (LV) systolic and diastolic function [Yamamoto T et al. J Am Soc Echocardiogr. 2003]. Tissue Doppler imaging is also useful for measuring atrial segment velocities and atrial strain rate for assessment of atrial function. Speckle tracking echocardiography is an angle independent tool for thorough assessment of LA performance but has not been validated for this use.
Echocardiography is considered an accurate modality for measuring LA size and function. 3DE is useful for guiding and monitoring interventions involving the atrial septum and LA appendage, while tissue Doppler imaging and speckle tracking echocardiography provide valuable information about atrial mechanics. Thorough evaluation of atrial size and function can improve the management of AF, refine risk stratification, and guide therapy.
Percentage of MRI-Detected Atrial Fibrosis Predicts AF Recurrence After Ablation
Atrial wall fibrosis and structural remodeling provide a substrate for the progression of AF and are associated with AF presence and persistence [Platonov PG et al. J Am Coll Cardiol. 2011]. Nassir F. Marrouche, MD, University of Utah Health Sciences Center, Salt Lake City, Utah, discussed the use of cardiac MRI for the detection of atrial fibrosis. According to Dr Marrouche, the MRI sequence used is key to the accurate evaluation of atrial fibrosis. His laboratory has developed protocols and pulse fibrosis sequences to optimize the images.
Challenges in MRI imaging of atrial fibrosis include acquisition errors and patient-related challenges. According to Dr Marrouche, acquisition errors include the wrong T1, wrong phase-encoding direction, partial coverage of the left atrium, errors in navigator prescription, and the wrong main frequency at 3T, as demonstrated in the Delayed-Enhancement MRI (DE-MRI) Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation (DECAAF) study [Marrouche NF et al. JAMA. 2014]. Patient-related challenges may include a high degree of heart rhythm irregularity, a high degree of nonregular respiration, rapid heart rate (> 120 bpm), and high body mass index (> 135 kg/m2).
A study of DE-MRI of the relatively load-independent measure categorized AF patients undergoing ablation according to structural remodeling stage based on the percentage of LA wall enhancement [McGann C et al. Circ Arrhythm Electrophysiol. 2014]. The DE-MRI result was validated with surgical biopsy. The study found that extensive LA wall enhancement predicted a poor response to catheter ablation for AF.
The Utah Staging System was developed to categorize patients according to the percentage of fibrosis quantified with DE-MRI, relative to the LA wall volume. It has been found that the percentage of patients with persistent AF increases with the degree of fibrosis found on DE-MRI, Dr Marrouche said. The risk of stroke has also been shown to increase with the degree of atrial fibrosis [Daccarett M et al. J Am Coll Cardiol. 2011].
Atrial fibrosis detected by DE-MRI in patients with AF was associated with recurrent arrhythmia after catheter ablation in the DECAAF trial [Marrouche NF et al. JAMA. 2014].
DE-MRI also detected ablation scarring 3 months postablation. The main predictor of recurrent AF after ablation was the percent of residual fibrosis, calculated as the amount of postablation scar plus fibrosis minus the scar. Dr Marrouche summarized his presentation with an algorithm for MRI-based (fibrosis-guided) patient selection and treatment strategy.
LA Strain Assessment in Management of AF
Erwan Donal, MD, Pontchaillou Hospital, Rennes, France, discussed the use of LA strain for assessing LA function in patients with AF. LA strain and strain rate are measures of local myocardial deformation that can be measured with tissue Doppler velocities or speckle tracking echocardiography. LA function consists of the reservoir function (filling pressure and LA compliance), conduit function (LV relaxation), and active contraction function (LV compliance, LV end-diastolic pressure, and LA intrinsic contractility).
LA volume can be assessed with 3D transthoracic echocardiography but measuring LA strain is more sensitive for assessing LA function. Speckle tracking improves the robustness of LA strain measurement. LA function assessment with speckle tracking takes 3.8 minutes, with 8% intraobserver variability and 9.5% interobserver variability [Paraskevaidis IA et al. Heart. 2009]. Global atrial systolic and diastolic strain rates are highly sensitive, are load dependent, and are related to LV longitudinal function as well as age [Boyd AC et al. Heart. 2011]. Table 1 describes results of studies of LA function assessed by atrial strain.
Studies of Left Atrial Function Assessed by Atrial Strain
Prof Donal concluded that atrial function can be evaluated by assessing the extent of LA remodeling with the speckle tracking approach in many clinical conditions. Expectations are high for the assessment of the atrial reservoir function with this approach as well. Further research and validation of speckle tracking are needed.
EAM Integrated With Other Modalities Produces Good-Quality Images and Reduces Radiation Exposure
Ole-Alexander Breithardt, MD, Heart Center, University Hospital, Leipzig, Germany, discussed the use of rotational LA angiography and 3D image-integrated fluoroscopy for AF ablation guidance. The LA anatomy varies from patient to patient. For that reason, the standard workflow includes anatomic imaging before the ablation procedure. This process involves using CT to create a 3D anatomic model, which is integrated with electroanatomical mapping (EAM) to improve the accuracy of the anatomical map. The workflow is complicated and time-consuming and often does not provide a completely accurate map.
Rotational 3D angiography (3DRA) is an alternative technique that can produce a 3D image of the LA during the ablation procedure. 3DRA is performed using cardiac C-arm CT with image integration into fluoroscopic views. Several studies of 3DRA have demonstrated quality images and lower radiation exposure with this technique.
A study evaluating 3DRA in different settings on ablation procedural aspects found that accuracy and radiation reduction were greatest with 3DRA and EAM integration [De Potter T et al. Arrhythmia & Electrophysiology Review. 2014].
A pilot study of a novel technique involving the real-time integration of fluoroscopy and real-time 3DE confirmed the technical feasibility of accurate real-time echo-fluoroscopic image overlay in clinical practice in patients undergoing AF ablation or transcatheter aortic valve implantation [Arujuna AV et al. IEEE J Translat Eng Health Med. 2014]. MRI-guided electrophysiology ablation is another novel technique currently under development.
Precise imaging of LA anatomy in patients with AF is a key factor for patient safety and successful ablation. 3D EAM is the basis for the ablation procedure, but is often insufficient for visualizing the complete and true anatomy. Registration of EAM with other modalities helps overcome these limitations and improves procedural outcomes, while reducing radiation exposure to patients and staff.
- © 2014 SAGE Publications