Summary
This article highlights new technologies that are currently in use or under investigation for 3-dimensional evaluation of the heart. These include a fusion imaging system between speckle tracking imaging and 64- to 320-slice multi-detector computed tomography, imaging intraventricular gradients and flow vectors, dynamic single-photon emission computed tomography, and 3-dimensional printing.
- tomography
- echocardiography
- intraventricular flow
- speckle tracking imaging
- left ventricular dysfunction
- SPECT
- D-SPECT
- 3D printing
In a session dedicated to new technologies, 4 speakers discussed novel approaches that allow evaluation of the heart in 3 dimensions.
Eduardo Casas Rojo, MD, University Hospital Ramon y Cajal, Madrid, Spain, discussed a newly developed 3D fusion imaging system: a combination of 3D ultrasound speckle tracking echocardiography (3D-STE) and 64- to 320-slice multidetector computed tomography (320 CT). Using this technology, hybrid images are produced from the separate patient data sets generated by the speckle tracking (Wall Motion Tracking) and computed tomography (CT) software programs. Prof Casas Rojo shared examples of these images, highlighting the use of this hybrid technology in patients with conditions such as left anterior descending (LAD) coronary artery stenosis, diagonal branch occlusion, and circumflex coronary (CX) artery stenosis [Casas Rojo E et al. Eur Heart J Cardiovasc Imaging. 2013]. However, he emphasized that since resting echocardiography is normal in many patients, stress echocardiography may be necessary.
According to Prof Casas Rojo, additional useful features of this hybrid technology have recently been recognized. These include its ability to evaluate individual coronary arteries, to examine stenosis, to set markers, and to measure strain in cases of stenosis of the LAD and CX arteries.
He also indicated a future application of this technology in evaluation of coronary veins and in cardiac resynchronization therapy, since 3D-STE allows visualization of the latest activated myocardial segments, and CT may also be adapted for enhancement of the coronary veins.
Mani A. Vannan, MBBS, Piedmont Heart Institute, Atlanta, Georgia, USA, discussed imaging intraventricular gradients and flow vectors. According to Dr Vannan, intraventricular flow is an important concept to consider in evaluating cardiac function. In the normal ventricle, he explained that early diastolic filling is categorized by a significant, noticeable vortex located beneath the anterior mitral valve leaflet that comprises an important hemodynamic mechanism for preserving myocardial efficiency. This vortex separates ventricular filling from ejection, and conserves energy during diastolic filling, which it subsequently imparts to the stroke volume leaving the ventricle during systole. In pathological conditions in which the vortex is disturbed, however, stroke volume is reduced, with an adverse effect on the heart’s pump function [Hong GR et al. JACC Cardiovasc Imaging. 2008]. Vortex hemodynamics can therefore predict long-term cardiac outcomes [Pedrizzetti G et al. Nat Rev Cardiol. 2014].
Intraventricular flow can be mapped using various methods, including echocardiography particle image velocimetry to track contrast particles with vectors. It is also now possible to map the morphology and location of the vortex structure, and examine its kinetic energy dynamics using a mapping algorithm. Using the example of a patient with dilated cardiomyopathy (DCM), Dr Vannan explained that the vortex in this case fails to reduce in size, indicating its inefficiency and failure to transfer its energy to the stroke volume leaving the ventricle during systole [Carlhäll CJ, Bolger A. Circ Heart Fail. 2010].
Abnormal vector momentum and direction in DCM is the trigger in a series of events that results in abnormal wall stress, he said. In a healthy individual, the vector direction travels from base to apex in a vertical direction. However, in patients with left ventricular (LV) dysfunction, Dr Vannan noted that the vector takes on a more horizontal and haphazard direction [Pedrizzetti G et al. Nat Rev Cardiol. 2014].
This abnormal vector momentum introduces a burden on the myocardium, resulting in abnormal wall stress and strain, leading to reduced ejection fraction, dilatation, and reduction in stroke volume.
Dr Vannan also shared data from a recent study that demonstrated 4D blood flow-specific changes in patients with DCM that may serve as markers of LV dysfunction [Eriksson J et al. Eur Heart J Cardiovasc Imaging. 2013]. In particular, patients with DCM had increased residual flow volume, which inflicts a greater strain on the myocardium.
According to Simona Ben-Haim, MD, University College London, London, United Kingdom, myocardial perfusion single-photon emission computed tomography (SPECT) is the main, noninvasive modality for detecting coronary artery disease (CAD) and evaluating its extent and severity in patients. Despite the superior accuracy of this well-established imaging technique, concerns exist about its radiation burden. Its lengthy acquisition time is also problematic, and needs to be optimized to increase patient comfort. However, Prof Ben-Haim emphasized that newer technologies with improved sensitivity and resolution have increased SPECT performance, providing faster myocardial perfusion imaging (MPI) with low radiation exposure. One such modality is dynamic single-photon emission computed tomography (D-SPECT), a novel high-speed dedicated cardiac camera technology using solid-state cadmium-zinc-telluride detectors to provide high image quality.
Prof Ben-Haim shared data from a recent study comparing ultra-low-dose imaging on a high-efficiency camera (D-SPECT) with standard-low-dose (SLD) imaging in 101 patients [Einstein AJ et al. J Nucl Med. 2014]. The results showed that D-SPECT imaging provided comparable perfusion and function to SLD imaging, with enhanced image quality and a radiation dose reduction to 1 mSv for a single injection.
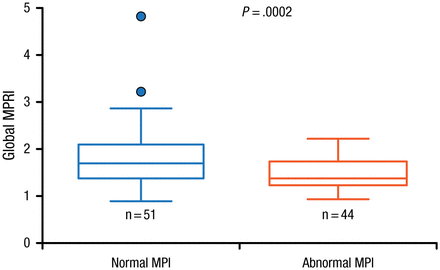
Since SPECT is also used to assess response to treatment in patients with CAD, and has roles in risk stratification, prognostication, and selection of patients for revascularization, Prof Ben-Haim identified the need for quantitative myocardial blood flow measurements. She noted that a major disadvantage of using conventional MPI is its underestimation of patient risk, particularly because its assessment of myocardial perfusion defects assumes that the best-perfused ventricular wall is normal. However, she discussed a recent study involving 95 patients that showed a role for D-SPECT imaging in the quantitative assessment of MPI and myocardial flow reserve [Ben-Haim S et al. J Nucl Med. 2013]. Data showed that the global myocardial perfusion reserve index (MPRI) was significantly higher (P = .0002) in patients with normal MPI (n = 51) compared with those with abnormal MPI (Figure 1).
Relationship Between Myocardial Perfusion Imaging and Myocardial Perfusion Reserve Index
Filled circles represent data points outside error bars.
MPI, myocardial perfusion imaging; MPRI, myocardial perfusion reserve index.
This research was originally published in JNM. Ben-Haim S et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: A feasibility study. J Nucl Med. 2013;54:873–879. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
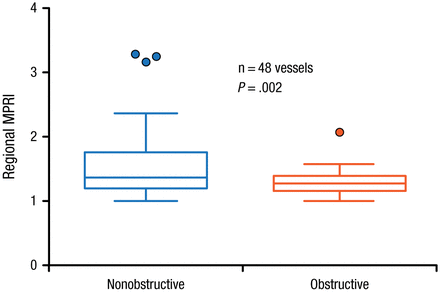
In patients who underwent invasive coronary angiography, the MPRI was significantly higher (P = .002) in territories supplied by obstructed compared with nonobstructed vessels (Figure 2) [Ben-Haim S et al. J Nucl Med. 2013].
Myocardial Perfusion Reserve Index in Association With Obstructed and Nonobstructed Vessels
Filled circles represent data points outside error bars.
MPRI, myocardial perfusion reserve index.
This research was originally published in JNM. Ben-Haim S et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: A feasibility study. J Nucl Med. 2013;54:873–879. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.
A 2010 study also demonstrated that the diagnostic performance of low-dose simultaneous dual-radionuclide (DR) D-SPECT MPI was comparable with sequential DR-D-SPECT using a conventional camera, with a significantly shorter imaging time [Ben-Haim S et al. Eur J Nuc Med Mol Imaging. 2010].
According to Prof Ben-Haim, D-SPECT is also emerging as a novel technology for imaging of the atria in patients with atrial fibrillation (AF). Studies are underway to evaluate its use in mapping the atrial sympathetic innervation, particularly with respect to imaging the epicardial and myocardial ganglionated plexi, and identification of hyperactive or nonreactive ganglia. It is also being used to map the viability and degree of scarring and fibrosis in the atria, and its use in catheter-ablation as an add-on to pulmonary vein isolation may facilitate treatment in patients with persistent AF.
Peter Verschueren, MSc, PhD, Materialise NV, Heverlee, Belgium, spoke about the application of 3D printing to cardiac imaging. Although 3D printing was originally used in prototype development in the automotive and aerospace industries, Prof Verschueren explained that it was introduced in the medical field about 2 decades ago. It was initially used to produce anatomical models, and later, surgical assist devices and implants, and was particularly employed in the field of orthopedic surgery to provide 3D reconstructions of a patient’s bone from a CT scan.
Prof Verschueren added that while it has only relatively recently been introduced in the field of cardiac imaging, it is particularly useful in complex cases that require multidisciplinary collaboration, such as between cardiac surgeons, interventional cardiologists, and anesthesiologists. In these instances, production of a 3D model of a patient’s heart allows doctors to plan a cardiac procedure prior to performing it.
He explained that imaging is the first step in 3D printing. Although technologies such as magnetic resonance imaging (MRI) and ultrasonography can be used, Prof Verschueren noted that CT provides the highest resolution images for 3D model production, although the use of MRI is on the rise. These images are subsequently used to generate 3D reconstructed models of the organ, and the technique uses a machine to build objects layer by layer, using plastics and other materials. He shared examples of the use of 3D printed models of the heart during his presentation, demonstrating their application in complex patient case planning, including in cases of congenital heart disease such as tetralogy of Fallot and ventricular septal defect.
He emphasized that although a printed heart model will contain the papillary muscles, it will not contain the cords or valve leaflets because these are difficult to see in the images and are too thin to print using the plastic materials involved in the technique.
In his concluding remarks, Prof Verschueren indicated that tissue engineering studies are underway to generate 3D cell culture scaffolds for production of heart models. He highlighted that progress in imaging technologies is also essential to improve the quality of 3D heart models, and that coordinated patient and economic studies are key to ensuring the correct use of this technology.
- © 2014 SAGE Publications