Summary
The four Janus kinases (JAKs), JAK1, JAK2, JAK3, and Tyk2, are essential components of the signaling pathway of certain cytokines [O'Shea JJ et al. N Engl J Med 2013]. This article reviews the role of JAKs in inflammation and JAK inhibitor efficacy and toxicity, the clinical data for tofacitinib, as well as discusses kinase inhibition in development, baricitinib, which strongly inhibits JAK1 and JAK2.
- Rheumatoid Arthritis
- Rheumatology
- Rheumatoid Arthritis
The four Janus kinases (JAKs), JAK1, JAK2, JAK3, and Tyk2, are essential components of the signaling pathway of certain cytokines [O'Shea JJ et al. N Engl J Med 2013]. John J. O'Shea, MD, National Institutes of Health, Bethesda, Maryland, USA, reviewed the role of JAKs in inflammation and JAK inhibitor efficacy and toxicity. JAKs transduce the signal from extracellular cytokines binding their transmembrane receptors by transferring phosphates from adenosine triphosphate (ATP) to substrates. JAKs are enzymes that mediate signal transduction. Many enzymes bind ATP but structural differences among these kinases provide different targets for specific kinase inhibitors. Dr. O'Shea pointed out that for any new kinase inhibitor, it is now possible to test its activity against all 518 protein kinases in the human kinome to determine specificity.
JAK inhibitors block only the type I and II cytokine receptors, which are dependent on Jaks for signal transduction. Type I and II receptor ligands include many interleukins (ILs), interferons (IFNs), and other molecules involved in the pathogenesis of rheumatoid arthritis (RA) and other autoimmune diseases; these also are relevant to the side effects of JAK inhibitors. Because JAK2 is essential for the function of erythropoietin, thrombopoeitin, and colony-stimulating factors, among other effectors, cytopenias are known to occur with first generation JAK inhibitors. Other possible toxicities include serious infections (eg, tuberculosis and herpes zoster), and increased lipids, which are of unknown significance. Dr. O'Shea mentioned that more selective JAK inhibitors are in development for a variety of autoimmune diseases. These are summarized in Table 1.
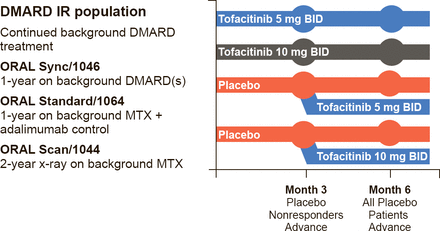
Iain B. McInnes, PhD, University of Glasgow, Glasgow, Scotland, United Kingdom, continued the discussion by reviewing the clinical data for tofacitinib, the first-in-class United States Food and Drug Administration (FDA)-approved JAK inhibitor. Tofacitinib mainly inhibits JAK3 and JAK1. The primary outcome measures for the registration trials included American College of Rheumatology 20% improvement response criteria (ACR20), Health Assessment Questionnaire Disability Index (HAQ-DI), Disease Activity Score (DAS28)<2.6, ACR 70% improvement response criteria (ACR70), and modified total Sharp score. The trial designs were similar, and those for Sync, Standard, and Scan are shown in Figure 1.
Specific JAK Inhibitors in Development for Autoimmune Diseases
Tofacitinib: Phase 3 Studies of 1 to 2 Year Duration—ORAL Sync, Standard, and Scan
DMARD=disease-modifying antirheumatic drug; IR=nonresponder; MTX=methotrexate.
Reproduced with permission from IB McInnes, PhD.
Patients were randomly assigned to 5 or 10 mg tofacitinib BID or placebo. Crossover was allowed at Month 3 for patients assigned to placebo whose disease did not respond; at Month 6 all patients assigned to placebo crossed over to active therapy. In Sync, Standard, and Scan, patients receiving either dose of tofacitinib had significantly improved ACR20, HAQ-DI, and DAS28 scores at Month 6, and the response was dose-dependent [Kremer J et al. Ann Intern Med 2013; van Vollenhoven RF et al. N Engl J Med 2012; van der Heijde D et al. Arthritis Rheum 2011 (abstr 2592)].
In two other Phase 3 studies, ORAL Step (with methotrexate) and ORAL Solo (monotherapy), patients were randomly assigned to tofacitinib 5 or 10 mg BID, or to placebo. At Month 3, all patients on placebo were randomly assigned to tofacitinib 5 or 10 mg BID. Results for these trials were similar to those for the other Phase 3 trials [Burmester GR et al. Lancet 2012; Fleischmann R et al. N Engl J Med 2012]. In the ORAL Solo trial, tofacitinib was associated with less fatigue and improved sleep compared with placebo [Strand V et al. Ann Rheum Dis 2011 (abstr 88)]. Responses in open-label extension therapy was maintained over 3 years. When compared with methotrexate, tofacitinib was associated with higher ACR70 rates over 12 months [Lee E et al. Arth Rheum (abstr 2486)], although in the current pharmacoeconomic environment, it is unlikely that tofacitinib would be used before methotrexate.
Over 4000 patients were involved in the registration trials, with about 7000 patient-years of exposure for the safety analysis. The rate of serious infection was about 3 per 100 patient-years, and was higher in patients receiving tofacitinib, particularly those who were aged >65 years, had diabetes, or were taking steroids or tofacitinib 10 mg. Infection was also associated with decreased lymphocyte counts, so tofacitinib should not be given to patients with a lymphocyte count <0.5 × 103/mm3. The rate of herpes zoster was higher than expected, so vaccination before the start of therapy is recommended. Tofacitinib was not associated with rate of malignancy higher than that reported in patients with RA, who are reported to have a higher rate of lymphoma than the general population [Curtis J et al. Arthritis Rheum 2013 (abstr 802)]. Lipid levels were increased at both doses of tofacitinib. However, less-atheroprotective components of high-density lipoprotein (HDL) decreased, and cardiovascular outcomes are similar for treatment with adalimumab, tofacitinib, or placebo [McInnes J et al. EULAR 2012 (abstr AB0160)]. Patients whose low-density lipoprotein (LDL) cholesterol increases can be treated with statins.
Joel M. Kremer, MD, Albany Medical College, Albany, New York, USA, concluded the session by discussing kinase inhibition in development, baricitinib, which strongly inhibits JAK1 and JAK2. Because of the JAK2 inhibition, side effects show a dose-dependent decrease in hemoglobin. In a Study in Participants With Rheumatoid Arthritis on Background Methotrexate [JADA; NCT01185353], although LDL increased with baricitinib, the number of particles decreased over 12 weeks of treatment; atherogenic very small particles decreased and protective large particles increased. HDL increased, and was also associated with protective particle size [Kremer J et al. Ann Rheum Dis 2013 (abstr THU0226)]. At 52 weeks of treatment on extension, responses were seen, although nonresponders were removed from the trial. Hemoglobin changes did occur, as did herpes zoster. One death due to myocardial infarction occurred, and the majority of patients experienced treatment-emergent adverse events [Taylor P et al. Ann Rheum Dis 2013 (abstr OP0047)].
Only early data are available for the other drugs in development, including GLPG0634 which targets JAK1; VX509, which targets JAK3; and ASP015K, which targets JAK1 and JAK2, and as shown in Table 1, trials are planned or ongoing.
Although JAK inhibitors are promising agents for autoimmune diseases, more research is needed to determine the right dose and schedule for any given patient. For each new agent it will be important to determine what other kinases in addition to JAKs are inhibited and the effect on efficacy and safety. There are multiple possible approaches to inhibit JAK targets, and the ideal JAK inhibitor with the best efficacy and toxicity profile for the treatment of RA has not been identified. Long-term trials will be necessary to determine the optimum dose and schedule for particular patients, but the large number of potential JAK inhibitors already identified suggests there will be future options for patients with RA whose disease does not respond to currently available treatments.
- © 2013 MD Conference Express®