Summary
Primary cardiac disorders in patients with connective tissue diseases are a consequence of the autoimmune process. Myocarditis evolving into dilated cardiomyopathy is a common complication of many connective tissue disorders, including systemic lupus erythematosus and systemic sclerosis. Other cardiac disorders associated with autoimmune diseases include endomyocarditis and endomyocardial fibrosis, pericarditis, valvulitis, hypertensive cardiomyopathy, and coronary arteritis. This article discusses the characterization, diagnosis, and management of these cardiovascular complications.
- Systemic Connective Tissue Disorders
- Imaging Modalities
- Lupus
- Inflammatory Disease
- Rheumatological Autoimmune Disorders
- Rheumatology
- Systemic Connective Tissue Disorders
- Imaging Modalities
- Lupus
- Inflammatory Disease
- Rheumatological Autoimmune Disorders
Primary cardiac disorders in patients with connective tissue diseases are a consequence of the autoimmune process. Myocarditis evolving into dilated cardiomyopathy (DCM) is a common complication of many connective tissue disorders, including systemic lupus erythematosus (SLE) and systemic sclerosis (SSc). Other cardiac disorders associated with autoimmune diseases include endomyocarditis and endomyocardial fibrosis, pericarditis, valvulitis, hypertensive cardiomyopathy, and coronary arteritis. The focus of this session was on the characterization, diagnosis, and management of these cardiovascular complications.
HEAVY CARDIAC DISEASE BURDEN IN PATIENTS WITH CONNECTIVE TISSUE DISORDERS
In his presentation, Yannick Allanore, MD, Rene Descartes University, Paris, France, discussed the features and diagnosis of primary cardiac disease in patients with SLE and SSc.
SLE affects most parts of the heart, including the pericardium, myocardium [Jain D, Halushka MD. J Clin Pathol 2009] and valves [Zuily S et al. Curr Rheumatol Rep 2013]. Pericardial involvement is found in 40% to 80% of patients with SLE on autopsy. Clinical symptoms manifest at disease onset or during relapse in 25% of patients and include chest pain with or without dyspnea, fever, tachycardia, decreased heart sounds, pericardial rubs, and, rarely, tamponade. Diagnostic tests include echocardiography and chest radiography. The standard treatments are nonsteroidal anti-inflammatory drugs, colchicine, steroids, and chronic immunosuppression [Jain D, Halushka MD. J Clin Pathol 2009].
Myocardial dysfunction is associated with myocarditis, drug-induced injury, or ischemic injury secondary to atherosclerosis. Patients are evaluated with coronary angiography or computed tomography to investigate coronary artery disease; if the results of these tests are negative, patients undergo magnetic resonance imaging (MRI) or endomyocardial biopsy. Patients generally are treated with high-dose corticosteroids and cyclophosphamide [Jain D, Halushka MD. J Clin Pathol 2009].
Pulmonary hypertension (PH) in patients with SLE develops as a result of conditions that cause hypoxic vasoconstriction, thromboembolism caused by antiphospholipid antibodies, pulmonary veno-occlusive disease, left heart disease, and noncirrhotic portal hypertension. Similar conditions lead to PH in patients with SSc [Dhala A. Clin Dev Immunol 2012]. Conduction defects and arrhythmias are common in patients with SSc; 30% to 50% of patients have abnormal results on resting electrocardiography (ECG). On Holter ECG, 20% have coupled ventricular extrasystoles, and 10% have nonsustained ventricular tachycardia. The risk for conduction defects is higher in patients with muscle and cardiac disease [Vacca A et al. Rheumatology (Oxford) 2014].
In summary, patients with SLE and SSc have a heavy burden of primary heart disease. Subclinical cardiac abnormalities are common in these populations.
CARDIAC MRI FOR DIAGNOSIS OF PRIMARY HEART DISEASE
Sven Plein, MD, PhD, University of Leeds & Kings College, London, United Kingdom, discussed the use of cardiac magnetic resonance (CMR) for diagnosing primary cardiac involvement in patients with autoimmune disease. Patients with rheumatoid arthritis (RA), SLE, and SSc are at risk for a variety of cardiovascular pathologies. According to Prof. Plein, CMR is accurate, reproducible, versatile, noninvasive, and mostly quantitative; uses nonionizing radiation; and is useful for tissue characterization. CMR has limitations, including limited availability, a requirement for expertise in acquisition and interpretation, and the mostly visual nature of analysis.
A study of CMR in patients with heart failure reported a marked improvement in reproducibility compared with 2-dimensional echocardiography [Bellenger NG, et al. J Cardiovasc Magn Reson 2000]. The first study to use CMR to assess cardiac structure and function in patients with RA found that the mean left ventricular mass was much lower in patients with RA compared with controls [Giles JT et al. Arthritis Rheum 2010].
Hachulla et al. [Ann Rheum Dis 2009] demonstrated that CMR is reliable and sensitive for diagnosing cardiac involvement in patients with SSc and for analyzing inflammatory, microvascular, and fibrotic components. CMR provided more information than echocardiography by visualizing inflammation and fibrosis. A study using T2-weighted CMR identified clinically unsuspected acute ischemic and inflammatory injury in patients with resuscitated sudden cardiac death and sustained monomorphic ventricular tachycardia [White JA et al. Circ Cardiovasc Imaging 2012].
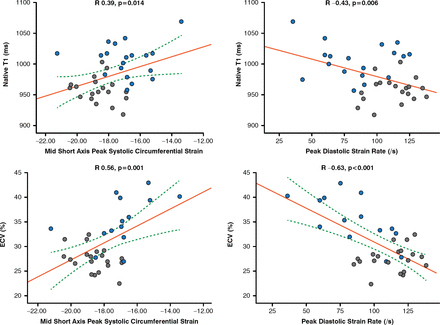
Another study used CMR with T1 mapping and extracellular volume quantification to visualize subclinical myocardial inflammation and diffuse fibrosis in patients with SSc [Ntusi NAB et al. J Cardiovasc Magn Reson 2014]. Native T1 and extracellular volume were significantly higher in patients with SSc versus controls (p<0.001) and were significantly correlated with disease activity and severity (Figure 1).
Correlation of Myocardial T1 and ECV to Peak Circumferential Systolic Strain and Peak Diastolic Circumferential Strain Rate
ECV=extracellular volume
Blue dot indicates systemic sclerosis; gray dot indicates control.
Reproduced from Ntusi NA et al. Diffuse myocardial fibrosis is subclinical and is associated with impaired myocardial deformation characteristics in systemic lupus erythematosus: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2014;16(Suppl 1):P307.
Table 1 lists the uses for the different CMR protocols in patients with autoimmune disease.
CMR Protocols and Their Uses
Prof. Plein concluded that CMR is a validated quantitative method for performing multiparametric assessment of cardiovascular morphology and function. Early data suggest a role for CMR in assessing cardiovascular involvement in autoimmune disease, but the studies have been small, and no outcomes data are available. Furthermore, many study results are nonspecific, and the potential exists for misinterpretation of data by inexperienced observers.
MANAGING CARDIAC COMPLICATIONS OF AUTOIMMUNE DISEASES
Alida L. P. Caforio, MD, PhD, University of Padova, Padova, Italy, reviewed the features, diagnosis, and management of cardiac involvement in patients with autoimmune connective tissue disorders. Autoimmune myocarditis is common among patients with these disorders and is defined as histologic myocarditis with negative viral polymerase chain reaction, with or without serum cardiac autoantibodies [Caforio ALP et al. Eur Heart J 2013].
Clinically suspected myocarditis is diagnosed by new abnormalities on ECG, Holter ECG, or stress testing; elevated myocardiocytolysis markers (troponin T or troponin I); functional and structural abnormalities on cardiac imaging; or tissue characterization by CMR [Caforio ALP et al. Eur Heart J 2013]. The European Society of Cardiology recommends treatment with immunosuppression for proven forms of autoimmune myocarditis, including giant cell myocarditis, cardiac sarcoidosis, and myocarditis associated with known extracardiac autoimmune disease [Caforio ALP et al. Eur Heart J 2013].
Table 2 shows the cardiac complications associated with autoimmune diseases and their treatments.
Cardiac Complications of Autoimmune Diseases
Many patients with systemic immune-related disorders develop inflammatory cardiomyopathy with or without a DCM phenotype. Clinically overt myocardial involvement confers a negative prognosis and may be life threatening. Subclinical inflammatory cardiomyopathy is likely to be underdiagnosed and inadequately treated. Furthermore, there is a lack of prospective studies using current cardiac imaging to define the frequency of inflammatory cardiomyopathy in the various autoimmune diseases. Prof. Caforio concluded that a multidisciplinary approach that includes a cardiologist is needed in the management of systemic immune-related disorders.
- © 2014 MD Conference Express®