Summary
A second catheter ablation is superior to antiarrhythmic drug (AAD) therapy for reducing the progression and prevalence of atrial fibrillation (AF) after an initial failed pulmonary vein isolation ablation for paroxysmal AF. In this randomized comparison of reablation and AAD therapy, progression to AF was substantial and progression to persistent AF not uncommon with AAD therapy but much less after redo ablation.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Cardiology & Cardiovascular Medicine
- Arrhythmias
A second catheter ablation is superior to antiarrhythmic drug (AAD) therapy for reducing the progression and prevalence of atrial fibrillation (AF) after an initial failed pulmonary vein isolation (PVI) ablation for paroxysmal AF. In this randomized comparison of reablation and AAD therapy, reported by Jonathan S. Steinberg, MD, Valley Health System, Columbia University, New York, New York, USA, progression to AF was substantial and progression to persistent AF not uncommon with AAD therapy but much less after redo ablation.
This was a prospective, randomized (1:1), active-controlled, parallel-arm trial in patients with recurrent symptomatic paroxysmal AF after a blanking period of initial PVI ablation procedure. An implantable loop recorder (ILR) was inserted in all patients. In the reablation arm, the endpoint of ablation was complete PVI at which point no additional ablation was undertaken unless an induced sustained atrial tachyarrhythmia (AT) was found. In the AAD arm, patients received either propafenone (450 to 900 mg/day), flecainide (200 to 400 mg/day), or sotalol (160 to 320 mg/day) at the discretion of the investigator instead of a second ablation.
Primary endpoint was the average AF burden on ILR calculated every 3 months. Secondary endpoints included freedom from recurrence of any AT (AF, flutter, etc), progression to persistent AF (≥7 days), progression of symptomatic AF prompting need for another ablation, and procedural complications and AAD adverse events.
Seventy-seven patients were randomized into each treatment arm. There were no baseline characteristic differences. Patient ages ranged between 49 and 64 years, 30% were hypertensive, most had a CHADS2 score of <1 and a left ventricular ejection fraction between 51% and −63%. The mean duration of AF was ∼4 years and the mean left atrial diameter was 45 mm. The majority (80%) of patient in the ADD arm received propafenone (mean 579±205 mg/day).
In the reablation group, PVI was accomplished in all 77 patients and no additional ablation was performed other than the repeat PVI. AAD therapy was discontinued in all patients at 6 weeks post ablation.
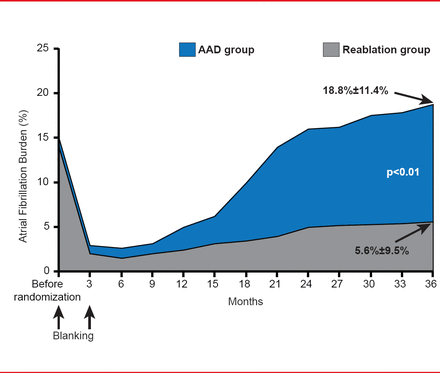
The baseline AF burden was similar (∼15%) for both groups. During the blanking period both groups experienced a dramatic decline in AF burden. After 3 to 6 months, the AF burden began to increase, and reached 18.8%±11.4% in the AAD group and 5.6%±9.5% in reablation group at 36 months. This difference was significant (p<0.01; Figure 1).
Primary Endpoint
AAD=antiarrhythmic drug.
Reproduced with permission from JS Steinberg, MD.
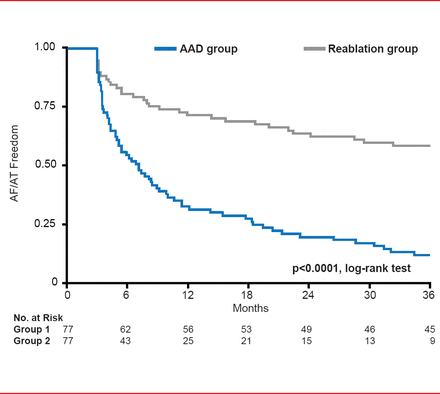
Freedom from AF/AT was significantly (p<0.0001) greater for the reablation group. At the end of the study only 12% of patients in AAD group were free of AF/AT compared with 60% in the reablation group (Figure 2).
Secondary Endpoint: Freedom From AF/AT
AAD=antiarrhythmic drug; AF=atrial fibrillation; AT=atrial tachyarrhythmia.
Reproduced with permission from JS Steinberg, MD.
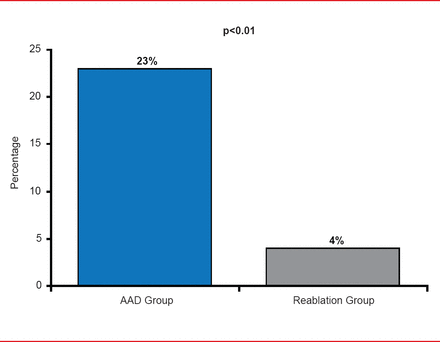
Progression to persistent AF was also significantly less (p<0.01) in the reablation group (4% of patients) versus 23% in the AAD group (Figure 3).
Secondary Endpoint: Progression to Persistent AF
AAD=antiarrhythmic drug; AF=atrial fibrillation.
Reproduced with permission from JS Steinberg, MD.
In the AAD arm, 64% (n=49) of patients discontinued therapy because of intolerance and/or inefficacy while 3% (n=2) patients experienced cardiac tamponade in the reablation arm.
Dr. Steinberg concluded by saying, “Reablation targeting restoration of PVI should be strongly considered when patients respond inadequately to the initial ablation.”
- © 2013 MD Conference Express®