Summary
Results of a first-in-man study of a novel intracardiac leadless catheter pacemaker (LCP) show its feasibility, safety, and efficacy for right ventricular (RV) pacing, reported study investigators. The prospective, nonrandomized, single-arm LEADLESS study [NCT01700244], conducted at three sites, evaluated the in vivo implantation of the LCP for the first time in 33 patients who required a permanent rate modulated ventricular-based pacemaker.
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Arrhythmias
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Arrhythmias
- Cardiology
Results of a first-in-man study of a novel intracardiac leadless catheter pacemaker (LCP) show its feasibility, safety, and efficacy for right ventricular (RV) pacing, reported study investigators.
Although conventional pacemakers are safe and effective, complications related to lead and generator pocket remain problematic. Each year, it is estimated that chronic lead-related problems affect 65,000 of the over 4.4 million people worldwide with pacemakers.
In an attempt to eliminate the complications related to the lead in conventional pacemakers, a novel percutaneously-delivered LCP was developed for implantation in the right ventricle with a battery life of at least 8 years.
In the prospective, nonrandomized, single-arm LEADLESS study [NCTO1700244] conducted at three sites, Vivek Reddy, MD, Mount Sinai School of Medicine, New York, New York, USA, and colleagues evaluated the in vivo implantation of the LCP for the first time in 33 patients who required a permanent rate modulated ventricular-based pacemaker. All patients included in the study had documented evidence of chronic atrial fibrillation (AF), normal sinus rhythm with second or third degree atrioventricular block and a low level of physical activity or short expected lifespan, or sinus bradycardia; were aged ≥18 years; had a life expectancy of at least 1 year; and were not pacemaker dependent.
Overall, the mean age of the patients included in the study was 75 years (range, 53 to 91 years), 64% were male, and most had sinus rhythm with low activity or short lifespan (60%) followed by chronic AF and second- or third-degree block (28%) and infrequent pauses or unexplained syncope (24%).
Implantation of the LCP was done by affixing the LCP to the endocardium with a single-turn helix, with a docking feature for repositioning and retrieval capability at the proximal end of the LCP. Implantation was done in the right ventricle by femoral venous access using a deflectable delivery catheter under x-ray guidance. Prior to release, baseline pacing and sensing thresholds were determined and the device was repositioned if these thresholds were suboptimal.
The pacemaker works by increasing the pacing rate with increased metabolic demand by sensing the RV blood temperature.
Evaluation of the primary endpoint (ie, safety) and secondary performance endpoints (ie, RV pacing function, battery longevity, rate response, implant success rate, and implant times) were done at 2 days, 2 weeks, 6 weeks, and 3 months after implantation.
Overall, 32 of the 33 (97%) of the patients were implanted successfully with the LCP. The time from procedure to hospital discharge was a mean of 1 day (range, 1 to 4 days).
The study found no major safety complications related to femoral access, with only one minor groin hematoma that did not require treatment. One patient also had a cardiac perforation and tamponade that was surgically repaired, but the patient had a large right-sided stroke 5 days after surgery and died.
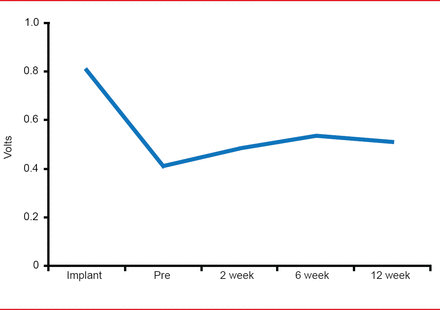
The performance of the device was demonstrated in data that showed pacing threshold changes over time (Figure 1), R-Wave amplitude changes over time, and impedance changes over time, that were similar to those expected to be seen in conventional pacemakers. Overall, pacing was achieved in ∼40% of patients.
Leadless Catheter Pacemaker Pacing Threshold Changes Over Time
Reproduced with permission from VY Reddy, MD.
Based on these results, the investigators concluded that leadless RV cardiac pacing is feasible and raises the possibility of eliminating lead from pacemakers. According to Dr. Reddy, commercial access of the LCP for clinical use is expected in Europe later this year, and a large multicenter study of the LCP in the United States is set to begin by sometime next year. In addition, an atrial LCP to allow for multichamber cardiac pacing is currently in development.
- © 2013 MD Conference Express®