Summary
Previous studies demonstrated that the structural changes to the heart that are associated with atrial fibrillation (AF) can be quantified using DE-MRI [Oakes RS et al. Circulation 2009]. This article presents data from the Delayed-Enhancement Magnetic Resonance Imaging (DE-MRI) Determinant of Successful Radiofrequency Catheter Ablation for Atrial Fibrillation trial [DECAAF; NCT01150214]. The DECAAF trial tested the hypothesis that DE-MRI can be used to determine the amount of left atrial fibrosis and/or remodeling, which predicts the patient's response to AF ablation.
- Cardiology Clinical Trials
- Arrhythmias
- Magnetic Resonance Imaging
- Cardiac Imaging Techniques
- Imaging Modalities
- Interventional Radiology
- Cardiology Clinical Trials
- Arrhythmias
- Magnetic Resonance Imaging
- Cardiology & Cardiovascular Medicine
- Cardiac Imaging Techniques
- Imaging Modalities
- Interventional Radiology
Nassir F. Marrouche, MD, University of Utah, Salt Lake City Utah, USA, presented data from the Delayed-Enhancement Magnetic Resonance Imaging (DE-MRI) Determinant of Successful Radiofrequency Catheter Ablation for Atrial Fibrillation trial [DECAAF; NCT01150214].
Previous studies demonstrated that the structural changes to the heart that are associated with atrial fibrillation (AF) can be quantified using DE-MRI [Oakes RS et al. Circulation 2009]. The DECAAF trial tested the hypothesis that DE-MRI can be used to determine the amount of left atrial fibrosis and/or remodeling, which predicts the patient's response to AF ablation.
In the international, prospective, multicenter, blinded, follow-up DECAAF trial, 330 patients that were undergoing their first AF ablation procedure were enrolled. Data from 261 patients were analyzed, as 57 MRIs were of poor quality and could not be analyzed and 12 patients were lost to follow-up. Patients were excluded from the study if they had a prior left atrial catheter or surgical ablation, were contraindicated for the DE-MRI contrast agent, were morbidly obese, and/or were too large for the MRI structure.
The follow-up period consisted of electrocardiogram, or Holter or event monitoring at 3, 6, and 12 months, as well as any additional follow-ups after 12 months, following the ablation procedure. The MARREK DE-MRI software sequence was installed at each participating center, and the staff was trained on five patients prior to enrolling for the DECAAF study. The primary endpoint for the DECAAF trial was recurrence of atrial arrhythmias following a 90-day blanking period.
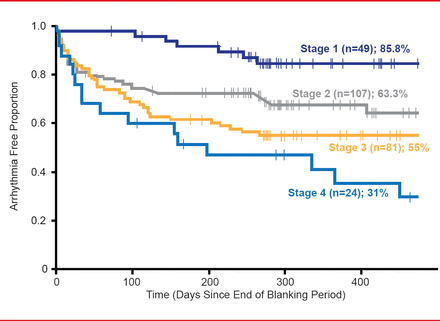
Quantification of atrial fibrosis by DE-MRI was categorized based on the Utah Classification System of Left Atrial Structural Remodeling, where stage 1 consists of <10% fibrosis/remodeling, stage 2 ≥10% to <20%, stage 3 ≥20% to <30%, and stage 4 ≥30% fibrosis/remodeling. The DECAAF trial results indicate that performing DE-MRI on patients prior to atrial ablation is feasible and produces reproducible data around the world. Interestingly, all patients in the trial had posterior wall involvement, which accounted for about 57% of the total fibrotic tissue in the left atrium. The only predictor of atrial fibrosis was history of hypertension.
Atrial fibrosis and/or structural remodeling, as quantified by DE-MRI, was demonstrated in multivariate analysis to be the only independent predictor of ablation outcome (Figure 1).
DE-MRI-Based Atrial Fibrillation Staging Is Associated With Ablation Procedure Outcome
Reproduced with permission from NF Marrouche, MD.
Dr. Marrouche concluded that, in his opinion, the data from the DECAAF trial indicates that DE-MRI quantification of atrial fibrosis is a very strong predictor of AF ablation outcome and can be reproduced around the world. He added that DECAAF data would promote DE-MRI based individualized management of the AF and help triaging patients to the appropriate treatment option based on the amount of atrial disease. In the future this would not only help procedural success, but also avoid unnecessary ablation procedures.
- © 2013 MD Conference Express®