Summary
Low-dose computed tomography (CT) is currently the best method for the early detection of lung cancer, and evidence demonstrates that screening saves lives when the technology is used properly for a high-risk population. However, lung cancer screening is not ideal, and issues include a high rate of false-positive results, complications related to diagnostic procedures, radiation-related harms, and patient anxiety. This article discusses the search for biomarkers, exhaled breath analysis, and airway gene-expression profiling, among other topics.
- Cancer
- Smoking Cessation
- Pulmonary Genomics
- Respiratory Cancers
- Oncology Genomics
- Oncology
- Pulmonary & Respiratory Medicine
Low-dose computed tomography (CT) is currently the best method for the early detection of lung cancer, and evidence demonstrates that screening saves lives when the technology is used properly for a high-risk population. However, lung cancer screening is not ideal, and issues include a high rate of false-positive results, complications related to diagnostic procedures, radiation-related harms, and patient anxiety, said Peter B. Bach, MD, MAPP, Memorial Sloan-Kettering Cancer Center, New York, New York, USA. In addition, the 20% reduction in mortality demonstrated in the National Lung Screening Trial (NLST) [NLST Research Team. N Engl J Med 2011] has not been reached in other studies [Infante M et al. Am J Respir Care Med 2009]. Reducing the cost and decreasing the potential harms of lung cancer screening and expediting the diagnosis of screening-detected nodules will help maximize the benefit of lung cancer. Researchers agree that better identification of people at high risk for the development of cancer is the way to achieve this goal.
As defined by the NLST, high risk describes a person who is aged 55 to 74 years, a heavy smoker (>30 pack-years), or a current or recent user (<15 years) of tobacco.
Care should be taken to define others who may benefit from lung cancer screening, said Douglas Arenberg, MD, University of Michigan, Ann Arbor, Michigan, USA, emphasizing the importance of estimating risk with a risk model. Dr. Arenberg added that physicians must be able to better define risk in order to discuss it with their patients. He noted that physicians must remember that mortality—not survival—is valid evidence of benefit of screening.
Dr. Bach said that when communicating risk to their patients, physicians should quantify the benefits and harms, using a round denominator (such as 1000) and absolute rather than relative numbers; emphasize not smoking; and recommend useful online tools to estimate risk.
To help better identify patients at risk for the development of lung cancer, researchers are exploring the use of biomarkers, and several innovative approaches show promise.
THE SEARCH FOR BIOMARKERS
Many biomarkers have been discovered, but none has reached clinical practice yet. Part of the problem is the long time needed for qualification and validation of candidate biomarkers, said Peter Mazzone, MD, MPH, Cleveland Clinic, Cleveland, Ohio, USA. Exhaled breath analysis, airway gene-expression profiling, and innovative technology to search for potential proteomic biomarkers are some steps toward improving the benefit of lung cancer screening.
EXHALED BREATH ANALYSIS
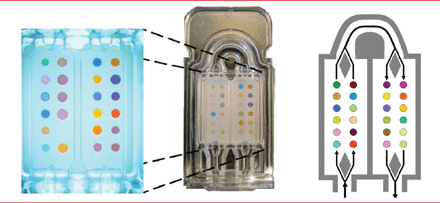
Dr. Mazzone explained that volatile organic compounds (VOCs) are present in the breath at low concentrations (low number per billion), and most VOCs represent metabolic processes in cells [Miekisch W et al. Clin Chim Acta 2004]. Research has shown that these processes differ between people with and without lung cancer. In a proof-of-principle study, colorimetric sensor array (Figure 1) had modest accuracy in detecting a unique chemical signature of the breath of people with lung cancer (sensitivity of 73.3% and specificity of 72.4%; p=0.01) [Mazzone PJ et al. Thorax 2007].
These findings were confirmed in a subsequent study with 229 subjects (92 with biopsy-proven lung cancer and 137 controls; 67 subjects at risk for the development of lung cancer and 70 subjects with benign lung nodules, 4 to 20 mm in diameter). The incorporation of clinical risk factors (age, sex, smoking history, and chronic obstructive pulmonary disease) led to greater accuracy of the analysis, as did a focus on only one histology [Mazzone PJ et al. J Thorac Oncol 2012]. Dr. Mazzone said that the colorimetric sensor array can be developed into a clinically useful tool, but the technique is still in the early phases of qualification. He added that it is unclear whether this technique will be more accurate than currently available screening and that its greatest benefits are its ease of use and the availability of real-time results.
AIRWAY GENE-EXPRESSION PROFILING
Avrum Spira, MD, Division of Computational Biomedicine, Boston University Medical Center, Boston, Massachusetts, USA, and colleagues have developed a gene-expression profile assay to test normal epithelial cells obtained with bronchial brushing. Airway gene expression reflects the physiologic response to tobacco smoke and can serve as an early diagnostic biomarker for lung cancer [Spira A et al. Nat Med 2007]. This biomarker may help address the problem of indiscriminate nodules detected on screening and challenges in decision-making after nondiagnostic bronchoscopy; for example, helping to determine whether a biopsy should be done or it is safe to wait for repeat imaging in 3 months.
Epithelial cells in the airway become exposed to toxins from cigarette smoke, leading to genomic abnormalities. Variability in response can be measured at the gene-expression level, and differences in response are associated with individual risk for the development of lung cancer. Approximately 80% of these gene-expression changes revert to normal after a smoker quits, but 10% to 20% are irreversible changes, persisting for years, sometimes decades, after quitting [Beane J et al. Genome Biol 2007].
Sensor Arrays
Note: Colorimetric sensor array for analysis of volatile organic compounds (VOCs) in exhaled breath. In the system, 36 spots composed of different chemically sensitive compounds are impregnated on a disposable cartridge. The colors of the spots change based on the chemicals with which they come into contact.
Reproduced from Mazzone P et al. Exhaled Breath Analysis with a Colorimetric Sensor Array for the Identification and Characterization of Lung Cancer. J Thorac Oncol 2012;7(1):137–142.
A 36-gene panel was used in a clinical validation test that distinguished smokers with and without lung cancer and was validated in an independent multicenter cohort. The sensitivity was high for nodules that were <3 cm and for stage I or II disease, with the sensitivity of the biomarker outperforming bronchoscopy (nodule <3 cm: 88% vs 66%; stage I or II disease: 86% vs 40%) [Whitney DH et al. ATS 2013 (poster k43)]. When the biomarker was combined with bronchoscopy, the sensitivities increased to 96% and 93%, respectively.
The next step is to create a less invasive test by analyzing RNA obtained from nasal mucosal brushings. The physiologic responses to smoking in the nose are similar to those seen in the bronchus, said Dr. Spira, with >90% of the genes in the nose changing in the same way in response to smoking as those in the bronchus [Zhang X et al. Physiol Genomics 2010]. Of smokers with suspect lung cancer, the test has an AUC of 0.73 in the diagnostic setting.
PROTEOMIC SEARCH FOR BIOMARKERS
Bloodborne biomarkers may also be useful in helping to identify people at high risk for lung cancer, but several physiologic and technical challenges are barriers, said Alessandra Luchini, PhD, George Mason University, Manassas, Virginia, USA. For example, bloodborne biomarkers associated with precancerous stages exist in very low concentrations, are obscured by abundant resident blood proteins such as albumin, and are rapidly degraded by endogenous and exogenous enzymes. The primary technical challenge is that mass spectrometry, the tool used most commonly to identify biomarkers, lacks sensitivity when applied directly to complex mixtures. Dr. Luchini has addressed these challenges with the development of a unique technology: bait functionalized hydrogel nanoparticles, which increase the sensitivity of mass spectrometry by four orders of magnitude.
This innovative nanotechnology was used in a study designed to discover and validate biomarkers for subclinical disease in blood samples obtained from initially healthy men up to 25 years before a lung cancer diagnosis. The biomarkers of interest were proteins found in the blood of healthy individuals in whom lung cancer subsequently developed, but not in the blood of individuals who remained cancer-free over the same follow-up period. The data set included samples from 40 people with adenocarcinoma and 40 matched controls. Dr. Luchini and coworkers determined the top-ranking proteins among smokers, former smokers, and never smokers and identified the proteins that best separated individuals with and without cancer in the three categories. Dr. Luchini noted that there was considerable overlap in the selected protein biomarkers in the smoker and former smoker categories and that the results suggest the mechanism of carcinogenesis may be different in nonsmokers. Selected proteins are now being validated in a new cohort of patients in a blinded fashion.
- © 2013 MD Conference Express®