Summary
The 2014 American College of Chest Physicians (CHEST) guidelines for cough, pulmonary arterial hypertension, acute exacerbations of chronic obstructive pulmonary disease, and mass critical care are discussed in this article.
- Chronic Obstructive Pulmonary Disease Thromboembolic Disease
- Pulmonary Guidelines
- Chronic Obstructive Pulmonary Disease
- Pulmonary & Respiratory Medicine
- Thromboembolic Disease
- Pulmonary Guidelines
Daniel Ouellette, MD, Henry Ford Hospital, Detroit, Michigan, USA, discussed the development of the 2014 American College of Chest Physicians (CHEST) guidelines, which were presented for cough, pulmonary arterial hypertension (PAH), acute exacerbations of chronic obstructive pulmonary disease (COPD [AECOPD]), and mass critical care. The 2014 CHEST guidelines follow other evidence-based guidelines concerning antithrombotic therapy, lung cancer diagnosis/management, pulmonary hypertension therapy, and COPD. The guidelines relied on the Institute of Medicine (IOM) standards that govern the formulation of clinical practice guidelines.
One standard is funding transparency. While prior CHEST guidelines were industry-funded, the current version was funded by CHEST. Evidence was evaluated considering the target population, intervention, comparative data, and outcomes. Other key IOM standards included rating strengths, systematic review, external review (typically part of the publication submission process), updating, and communication of recommendations to concerned clinicians and others.
The process of guideline development from establishment of the panel to the submission of the recommendations for publication takes about 14 months. At any time, a host of guidelines addressing specific areas can be in different phases of development. Each guideline development group should be a balance of topic experts, methodology experts, clinicians, and selected and unbiased consumer representatives and should have patient/public involvement. An ongoing part of any guideline development panel is the disclosure of any existing/new pertinent conflict of interest and if necessary, exclusion of the conflicted panelist.
In instances where formulation of clinical practice guidelines necessitates a systematic review of the literature, attention should be paid to the use of existing reviews judged to be acceptable and in-house reviews. Each recommendation involves ≥ 2 panelists, ≥ 1 of whom has no pertinent conflict of interest; that person writes the recommendation/suggestion. Rating the strength of the evidence involves a description of the benefits and harms, ratings of the level of confidence and strength of each recommendation, and a description of any differences of opinion during recommendation formulation. The result is a rating scale from 1A to 2C (Table 1).
Strength of Evidence of the CHEST Recommendations
Of the adopted recommendations, which are voted on anonymously, strongly rated recommendations should be worded in a way that allows compliance to be monitored.
COUGH GUIDELINES
Richard Irwin, MD, University of Massachusetts, Worcester, Massachusetts, USA, described an interim report of the CHEST cough guideline. The latest version continues the evidence-based format that extends back to 1998. Then as now, cough is the most common reason for ambulatory medical care in Americans. By 2006, the year of the last CHEST cough guideline, > 12 countries had formulated and published cough guidelines.
The goals of the 2014 CHEST cough guideline were to review new developments in the intervening years, update information according to the IOM guidelines, and identify topics of clinical/research importance. The latter includes acute cough (acute bronchitis and allergic rhinitis), subacute postinfection cough, and chronic cough due to a variety of causes; cough in special patient groups; and symptomatic treatment using cough suppressants and pharmacologic therapy. The guideline provides an overview of cough management and associated methods, cough anatomy/neurophysiology, assessing outcomes of studies of chronic cough, and classification of cough. Over a dozen other topics are nearing publication or are in various stages of development.
MANAGEMENT OF PULMONARY ARTERIAL HYPERTENSION IN ADULTS
Darren Taichman, MD, PhD, University of Pennsylvania, Philadelphia, Pennsylvania, USA, discussed an updated pharmacological therapy guideline for adult PAH [Taichman DB et al. Chest. 2014]. Treatment must be preceded by an accurate diagnosis involving echocardiogram, blood work, assessed lung function, imaging, and cardiac catheterization; Dr Taichman stressed that since treatment for PAH is not the same as for other forms of pulmonary hypertension, treatment before accurate diagnosis is irresponsible.
As guideline standards have changed, the 2014 guideline differs from the previous, 2007 version. Recommendations now need to be based on ≥ 2 randomized controlled trials, using pooled data that is consistent in both the interventions and the outcomes. As well, evidence is evaluated and downgraded if it is judged to be indirect, inconsistent, or imprecise.
The result can be a less-than-optimal evidence base, but clinicians still require guidance. Thus, the 2014 document is a hybrid, with evidence-based recommendations as warranted, as well as consensus-based (CB) statements. The guideline approach is based on World Health Organization (WHO) functional classes (FC) (Table 2).
WHO Functional Classification
The available evidence does not provide a clear, simple treatment algorithm. Rather, the severity of PAH should be evaluated on a case-by-case, systematic, and consistent basis using a combination of the WHO FC, patient capacity for exercise, possible benefits and adverse effects of the available drugs, clinician judgment, and data from assessments including echocardiograms. Assessments should involve a center with expertise in the diagnosis of PAH.
Patients without right heart failure who demonstrate acute vasoreactivity can be treated using an oral calcium channel blocker (CCB); however, CCB treatment should not be given empirically, in the absence of vasodilator test results. Treatment-naïve asymptomatic patients and those at increased risk of developing PAH should be monitored for symptoms that trigger treatment.
Treatment-naïve patients with WHO FC II symptoms who are contraindicated for or have failed CCB therapy should be treated with monotherapy. Suggestions were graded on the 1A to 2C scale above or as CB, and include ambrisentan (Grade 1C), sildenafil (Grade 1C), riociguat (Grade CB), or tadalafil (Grade CB) to improve 6-minute walking distance; riociguat (Grade CB) or macitentan (Grade CB) to delay time to clinical worsening; and bosentan or riociguat to improve cardiopulmonary hemodynamics. Parenteral or inhaled prostanoids should not be chosen in this population.
Treatment-naïve patients with WHO FC III symptoms who are contraindicated for or have failed CCB therapy should also be treated with monotherapy, with bosentan suggested to decrease hospitalization due to PAH.
Treatment-naïve PAH patients with WHO FC III symptoms and evidence of rapid disease progression or markers of poor clinical prognosis, or FC IV patients, can be treated initially with a parenteral prostanoid: continuous intravenous epoprostenol or treprostinil, or continuous subcutaneous treprostinil.
Combination therapy can be considered in some cases. PAH patients who remain symptomatic when receiving stable doses of endothelin-receptor antagonist or phosphodiesterase-5 inhibitor can benefit from the addition of inhaled iloprost or treprostinil. Those who are symptomatic with stable doses of intravenous epoprostenol may benefit from additional sildenafil.
Prudent strategies for PAH patients include maintaining current vaccinations, avoiding pregnancy, avoiding unnecessary surgery, and ensuring a supplemental supply of oxygen when flying. PAH remains incurable and disease progression is inevitable. Evidence is still sparse, which continues to make treatment challenging.
PREVENTION OF ACUTE EXACERBATIONS OF COPD
Gerard J. Criner, MD, Temple University School of Medicine, Philadelphia, Pennsylvania, USA, described prevention of AECOPD, defined as an event requiring intervention using antibiotics and/or systematic steroids. The focus on AECOPD is important, since the acute events can diminish both lung function and quality of life, and herald increased risks of mortality and morbidity.
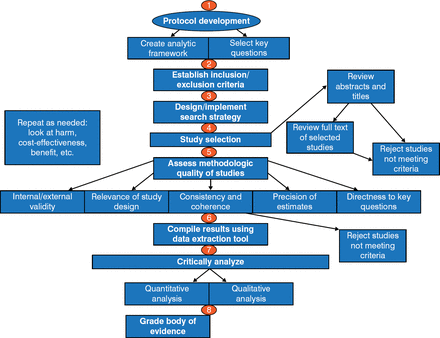
The goal of the 2014 evidence-based AECOPD guideline formulated by CHEST and the Canadian Thoracic Society (CTS) was to describe the current state of knowledge regarding AECOPD, with selection of the highest quality evidence [Criner GJ et al. Chest. 2014]. The process was robust and detailed (Figure 1).
Evidence Review Process in the 2014 CHEST-CTS Guideline
Reproduced with permission from GJ Criner, MD.
Evidence was graded with a detailed consideration of the relative value of the treatment benefits and risks/burdens, specific to whether nonpharmacological therapy, pharmacological inhaled therapy, or pharmacological oral therapy prevented or decreased AECOPD. The quality of evidence was judged as high, moderate, or low using defined criteria.
Recommendations concerning nonpharmacological therapies included annual influenza vaccination, pulmonary rehabilitation within 4 weeks of the event, and patient education, management, and follow-up. Suggestions include pneumococcal vaccination and stopping smoking.
Recommendations for pharmacological inhaled therapy include long-acting β-agonists, long-acting muscarinic antagonists, and corticosteroids. Suggested therapy includes short-acting muscarinic antagonists in combination with short- or long-acting β-agonists.
Suggested pharmacological oral therapies include long-term macrolides, phosphodiesterase-4 inhibitors, theophylline, N-acetylcysteine, and carbocysteine. The use of systemic corticosteroids and statins is not recommended.
MASS CRITICAL-CARE EVENTS
Mike Christian, MD, MSc, Mount Sinai Hospital, Toronto, Ontario, Canada, provided a high-level overview of the recently published CHEST Consensus Statement concerning mass critical care, specifically care of the critically ill and injured during disasters and pandemics [Christian MD et al. Chest. 2014]. The current example of mass critical care is the Ebola outbreak in western Africa, which at the time of the conference exceeded 10 000 cases.
The CHEST supplement, comprising 18 sections, included a consensus statement on surge capacity logistics, which is the capability of providing mass critical care in times of disaster or a pandemic. Stockpiling of equipment, supplies, and pharmaceuticals is crucial for the swift implementation of mass critical care, as is a plan to utilize transportation routes. Hospitals that could be involved in mass critical care also need to be prepared for triage and potential evacuations. It is important to identify and remedy weaknesses in the supply chain. Equally important is IT support to ensure continued flow of health information during times of disruption and relocation. Finally, Dr Christian noted that although the section on infrastructure and capacity-building in resource-poor settings was initially considered as almost an afterthought, the information it contains that could aid in dealing with the next big outbreak has brought greater interest.
- © 2014 MD Conference Express®