Summary
Treatment of chronic bronchopulmonary infection by Pseudomonas aeruginosa in patients with cystic fibrosis (CF) with liposomal amikacin for inhalation (LAI) resulted in improved pulmonary function and decreased P aeruginosa sputum concentration in an interim analysis. This article discusses interim data from the Long Term Safety and Tolerability of Open-Label Liposomal Amikacin for Inhalation in Cystic Fibrosis Patients With Chronic Infection due to Pseudomonas aeruginosa trial [CLEAR-110; NCT01316276; Bilton D et al. Am J Respir Crit Care Med 2014].
- Lower Respiratory Infections
- Pulmonary Clinical Trials
- Lower Respiratory Infections
- Pulmonary Clinical Trials
- Pulmonary & Critical Care
Treatment of chronic bronchopulmonary infection by Pseudomonas aeruginosa in patients with cystic fibrosis (CF) with liposomal amikacin for inhalation (LAI) resulted in improved pulmonary function and decreased P aeruginosa sputum concentration in an interim analysis. Diana Bilton, MD, Royal Brompton Hospital, London, United Kingdom, presented interim data from the Long Term Safety and Tolerability of Open-Label Liposomal Amikacin for Inhalation in Cystic Fibrosis Patients With Chronic Infection due to Pseudomonas aeruginosa trial [CLEAR-110; NCT01316276; Bilton D et al. Am J Respir Crit Care Med 2014].
The study treatment consists of amikacin formulated within biocompatible liposomes of about 0.3 µm, which are able to penetrate the biofilm formed by P aeruginosa and nontuberculous mycobacteria. The CLEAR-108 study showed that LAI treatment resulted in similar forced expiratory volume in 1 second (FEV1) in patients with cystic fibrosis and chronic P aeruginosa infection as compared with tobramycin inhalation solution (TIS). Patients who completed CLEAR-108 were eligible to enroll in the extension study, CLEAR-110. The purpose of the CLEAR-110 study was to determine the long-term safety and efficacy of LAI.
In CLEAR-110—a multicenter, open-label, Phase 3 study—206 patients aged ≥6 years received 6 cycles of LAI (28 days on, 28 days off) in the first extension and an additional 6 cycles in the second extension. Patients were assessed monthly by the Cystic Fibrosis Questionnaire– Revised, colony-forming units, need for antibiotic rescue treatment, pulmonary exacerbation, and hospitalizations. The patients had a mean age of 21 years and a
mean FEV1 percent predicted of 65.5. The primary outcome measures were the relative change in FEV1 and FEV1 percent predicted, the incidence of treatment-emergent adverse events, and acute tolerability. Secondary efficacy outcomes included a relative change in FEV1 and FEV1 percent predicted, time to first protocol-defined pulmonary exacerbation, time to first antipseudomonal antibiotic treatment, shift in minimum inhibitory concentration, and evaluation of emergent pathogens.
Common (>10%) treatment-related adverse events included infective pulmonary exacerbations of cystic fibrosis, nasopharyngitis, upper respiratory tract infection, hemoptysis, cough, and dysphonia. Most adverse events were mild to moderate in severity, and none of the patients receiving LAI discontinued the study drug due to treatment-related adverse events.
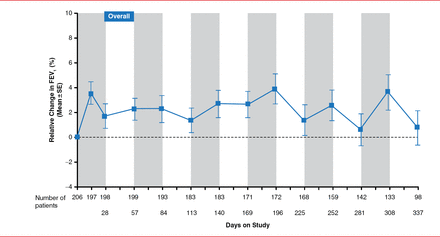
Treatment with LAI resulted in a sustained improvement in FEV1 from baseline over the study period (Figure 1). In addition, patients enrolled in CLEAR-110 who had received TIS in the CLEAR-108 trial showed an increase in FEV1. Similarly, patients who had received LAI in the CLEAR-108 trial experienced sustained improvement in FEV1 during LAI treatment in the CLEAR-110 trial.
Effect of Long-Term LAI Treatment on FEV1
Note: Shaded regions represent 28 days on-treatment; dashed line represents baseline.
FEV1=forced expiratory volume in 1 second; LAI=liposomal amikacin for inhalation.
Reproduced with permission from D Bilton, MD.
The density of P aeruginosa in sputum appeared to generally decrease from baseline levels, regardless of treatment assignment in CLEAR-108.
In conclusion, Dr. Bilton stated that, in her opinion, the data from the CLEAR-110 extension trial suggest that LAI was well tolerated in patients with cystic fibrosis and chronic infection by P aeruginosa. In addition, LAI treatment may improve long-term pulmonary function. Dr. Bilton also pointed out that this interim analysis did not include the entire study population, as the trial is ongoing; therefore, conclusions of the data cannot yet be determined.
- © 2014 MD Conference Express®