Summary
Cervical cancer remains a major public health problem worldwide. Current data show that worldwide, there are >500,000 new cases of cervical cancer and >275,000 deaths annually. As many as 85% of the new cases and deaths are occurring in developing countries, where it is the first- or second-leading cause of cancer and related deaths among women [Siegel R et al. CA Cancer J Clin 2013; Globocan 2012]. This article reviews screening and vaccination approaches for cervical cancer prevention.
- Vaccinations
- Reproductive Cancers
- Sexually Transmitted Diseases
- Vaccinations
- Reproductive Cancers
- Sexually Transmitted Diseases
- Reproductive Cancers
- Obstetrics & Gynecology
Cervical cancer remains a major public health problem worldwide, stated Michael Maxwell Frumovitz, MD, MD Anderson Cancer Center, Houston, Texas, USA, who reviewed screening and vaccination approaches for its prevention.
Current data show that worldwide, there are >500,000 new cases of cervical cancer and >275,000 deaths annually. As many as 85% of the new cases and deaths are occurring in developing countries, where it is the first- or second-leading cause of cancer and related deaths among women [Siegel R et al. CA Cancer J Clin 2013; Globocan 2012]. Some 3.4 million women-years of life are lost annually to cervical cancer. In the United States, public health efforts have successfully reduced cervical cancer by about 70% with Papanicolaou (Pap) test screening over the past 40 years, and it is now the 14th most frequent cancer among women, with approximately 12,000 new cases and 4,000 deaths annually.
PREVENTION OPPORTUNITIES
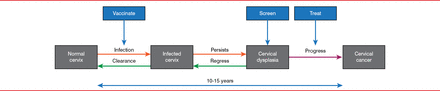
The natural history of cervical cancer provides multiple opportunities for prevention (Figure 1). Cervical infection by human papillomavirus (HPV) is the genesis of cervical cancer, causing a local infection on the cervix. Most immunocompetent young women (about 90%) will clear an HPV infection in 2 years [Ho et al. N Engl J Med 1998]. A persistent infection leads to cervical dysplasia, which will regress without intervention in about 70% of cases of low-grade dysplasia and in some cases of high-grade dysplasia. A small number of lesions will progress from low grade to high grade and eventually to cervical cancer, a process that generally takes 10 to 15 years. Dysplasia can be treated with surgical resection, laser ablation, and cryotherapy, among other accepted techniques, while emerging technologies and immunotherapy are being explored.
Opportunities to Intervene to Prevent Cervical Cancer
Reproduced with permission from Michael Maxwell Frumovitz, MD.
VACCINATION TO REDUCE CERVICAL CANCER
HPV is the most common sexually transmitted disease, and the initial infection usually occurs during adolescence, within 18 months of sexual debut. Notably, 80% of women will have HPV infections during their lifetimes, of whom <5% will have significant preinvasive disease and <1% will have invasive cervical cancer [Centers for Disease Control and Prevention 2004; Baseman JG, Koutsky LA. J Clin Virol 2005].
The 2 approved vaccines have an efficacy of 93% to 100% against high-risk HPV types in adolescent girls between 16 and 18 years of age [Garland SM et al. N Engl J Med 2007; The FUTURE II Study Group. N Engl J Med 2007; Lehtinen M. Lancet Oncol 2012].
Table 1 summarizes the recommendations for vaccination of 4 key medical societies.
Recommendations for HPV Vaccination*
Vaccination of adolescent boys (with recombinant HPV quadrivalent [types 6,11,16, and 18] vaccine) is also recommended to prevent genital warts and potentially to prevent other HPV-associated cancers (anal, penile, or oropharyngeal). HPV is estimated to be the cause of about 75% of oropharyngeal cancers. Furthermore, vaccination of boys is thought to decrease HPV transmission to female partners and to provide greater protection of the public by decreasing infection in those who do not receive vaccination.
The impact of vaccination was estimated using mathematical models of the data from the Papilloma Trial Against Cancer in Young Adults trial [Van Kriekinge G et al. Vaccine 2014]. Vaccination of 70% of adolescent girls worldwide would lead to a 50% reduction in cancers associated with HPV-16 and HPV-18, a reduction of 345,000 new cases of overall cervical cancer, and the prevention of 179,000 deaths due to cervical cancer [Van Kriekinge G et al. Vaccine 2014].
A mandatory vaccination program of schoolgirls in Australia with a quadrivalent vaccine provides real-world data on the benefit of vaccination and shows that a strong nationwide vaccination program reduces the morbidity and eventually the mortality associated with cervical cancer, said Dr. Frumovitz. A 90% reduction in reports to national health authorities was observed within 2 years of the initiation of the mandatory HPV vaccination program in Australia [Fairley CK et al. Sex Transm Infect 2009]. Another study showed a 40% reduction in high-grade dysplasia in women younger than 18 years of age within 3 years of the initiation of the mandatory Australian program [Brotherton JM et al. Lancet 2011].
Rates of HPV vaccination range from >70% in Australia, Canada, the United Kingdom, and Panama to only 33% of girls and 7% of boys completing the 3-dose series in the United States [Morbid Mortal Wkly Rep 2013].
RECOMMENDATIONS FOR CERVICAL CANCER SCREENING
Screening for cervical cancer is highly effective, stated Dr. Frumovitz, as shown by the 70% reduction in cervical cancer at 7 years after a national screening program began in England in 1988 [Quinn et al. BMJ 1999]. Screening is necessary because of the low uptake of HPV vaccination and because it does not cover all the high-risk subtypes.
Three governing bodies updated their recommendations for cervical cancer screening in 2012. These were the joint American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology guidelines [Saslow D et al. J Low Genit Tract Dis 2012]; the American Congress of Obstetricians and Gynecologists [Committee on Practice Bulletins—Gynecology. Obstet Gynecol 2012]; and the US Preventive Services Task Force [Moyer VA. Ann Intern Med 2012].
For women at average risk, screening should begin at 21 years of age and continue at 3-year intervals through age 29 years. For women ages 30 to 65 years, the guidelines are in agreement that screening should take place every 5 years with both cervical cytology and HPV testing and that cervical cytology alone every 3 years is acceptable. For women older than 65 years who had previous abnormal Pap smear results, screening should continue until there are 15 to 20 years of normal Pap smear results, even past the age of 65 years. In the future, with more data on screening efficacy, it may be that cervical cytology will be used to determine which HPV-positive women should be followed or undergo colposcopy.
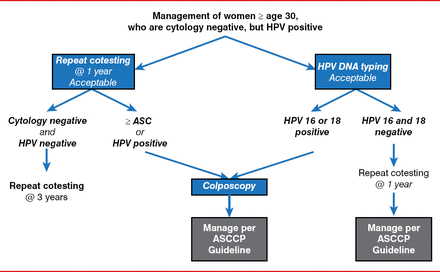
The American Society for Colposcopy and Cervical Pathology's management algorithm for screening women 330 years of age who are HPV positive and have normal Pap smear results is shown in Figure 2 [Massad LS et al. Obstet Gynecol 2013; Saslow D et al. J Low Genit Tract Dis 2012].
American Society for Colposcopy and Cervical Pathology Screening Algorithm
ASC=atypical squamous cells; ASCCP=American Society for Colposcopy and Cervical Pathology; DNA=deoxyribonucleic acid; HPV=human papillomavirus.
Reproduced with permission from Lippincott, Williams & Wilkins from Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis 2012;16(3):175–204.
The use of Pap smears for screening is limited by their low sensitivity and specificity, resulting in high rates of false-negative and false-positive results, respectively, stated Dr. Frumovitz. This has led to screening strategies that now start with HPV testing. One such strategy recommends routine screening for HPV-negative patients, while HPV-positive patients should undergo reflex cytology with or without HPV genotyping to determine the need for further screening or colposcopy [Wright TC Ir. Clin Ob Gyn 2007].
HPV testing is more sensitive and reproducible than Pap testing [Mayrand MH et al. N Engl J Med 2007], is more upstream in the carcinogenic process, can be automated and centralized, and is more cost-effective, stated Dr. Frumovitz. Based on data from the Addressing the Need for Advanced HPV Diagnostics trial [Cox IT et al. Am J Obstet Gynecol 2012; Castle PE et al. Lancet Oncol 2011], the US Food and Drug Administration recently approved a new screening tool (cobas HPV Test) for cervical cancer screening said Dr. Frumovitz.
- © 2014 MD Conference Express®