Summary
Dr. Kenneth Offit, MD, MPH, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical College, New York, New York, USA, received the 2013 ASCO-American Cancer Society Award. In his lecture on the use of genetic information as a tool for cancer prevention, Dr. Offit discussed the enormous social, ethical, and legal challenges in the field of oncology, and further highlighted opportunities for change in the practice of medicine.
- Oncology Genomics
- Exclusive Article - For home page
- Oncology Genomics
- Oncology
- Exclusive Article - For home page
Dr. Kenneth Offit, MD, MPH, Memorial Sloan-Kettering Cancer Center, Weill Cornell Medical College, New York, New York, USA, received this year's ASCO-American Cancer Society Award. In his lecture on the use of genetic information as a tool for cancer prevention, Dr. Offit discussed the enormous social, ethical, and legal challenges in the field of oncology, and further highlighted opportunities for change in the practice of medicine.
One of Dr. Offit's most significant contributions to the field was the identification of the BRCA2 mutation (617delT) in Ashkenazi Jewish women affected by breast and ovarian cancers [Neuhausen S et al. Nat Genet 1996]. This finding is the single most common genetic mutation associated with a highly penetrant form of cancer. However, the finding applies to women beyond the Ashkenazi population.
Changes in individual and population behaviors can help reduce—or even prevent—a significant number of cancers. Together, smoking and obesity account for 53% of this potential reduction, while hereditary factors account for only 16% of cancers. Integrating current knowledge about genetic factors into targeted prevention efforts could ultimately reduce the rate of hereditary-related cancers by half.
Sequencing tumors to target therapy will allow focused cancer prevention in families.
Much of our disease knowledge has come from the hereditary history of families. Despite the work of Gregor Mendel, the existence of hereditary cancer was at first considered of little value. Most of Mendel's genetic work laid in obscurity until the 1930s. In 1971, Henry Lynch was the first to identify a family history of breast and ovarian cancer and advance the idea that cancer arises from gene mutations—lending some credibility to Mendel's theory.
“All cancer is a genetic disease….but how much is hereditary?” questioned Dr. Offit. Two studies have reshaped the thinking around this question: the Utah Genealogical “Experiment” and the Scandinavian twin registry. The Utah study tracked the genealogical records of Brigham Young's 2 million descendents from the time he moved his original 10,000 followers to Salt Lake City, Utah. The Utah Population Database used these data to determine the various genetic relationships among the 2 million individuals, while the Genealogical Index of Familiarity measured the genetic links between cancer cases statewide. The data uncovered unusually high levels of familial clustering of cancer, specifically lymphocytic leukemias, and especially chronic lymphocytic leukemia, lobular breast cancer, early-stage lip cancer, early-stage melanoma, and female lung cancers of alveolar/adenoma histology [Cannon-Albright LA et al. Cancer Res 1994].
The twin study examined data on 44,788 pairs of twins listed in the Swedish, Danish, and Finnish twin registries to assess the role of inherited genetic factors in the development of malignant diseases. Statistically significant genetic effects were observed for prostate, colorectal, and breast cancer [Lichtenstein P et al. N Engl J Med 2000]. These findings were confirmed using the concept of a “genometype” in a large number of monozygotic twin pairs. A specific genometype represents the genomes in the population conferring a specific level of genetic risk for a specified disease [Roberts NJ et al. Sci Transl Med 2012].
The discovery of BRCA mutations in ancestral groups may be explained by the Founder Effect. After the Jewish pogroms in Russia, mutations become more concentrated in individuals belonging to groups with marked population decreases. When the group began to repopulate, the mutation spread as a result of low genetic variation.
The value of personalized genomics medicine in cancer prevention was first demonstrated in a study of BRCA mutation carriers who underwent a risk-reducing salpingo-oophorectomy (RRSO). The study found that undergoing RRSO can reduce the risk of breast cancer and BRCA-related gynecologic cancer in BRCA carriers [Kauff ND et al. N Engl J Med 2002].
Advances in science and technology are revolutionizing approaches to genetic cancer risk assessment and prevention, but innovation does not come without complications. An example is the “Myriad Case” recently under review by the United States Supreme Court. Myriad Genetics claimed to own the rights to any test for the presence of the BRCA1 and 2 genes, as well as patents on the methods for interpreting the test results; however, a unanimous ruling by the court in June 2013 bars the patenting of any naturally occurring genes. Evidence-based information regarding the clinical utility of genome testing is needed, along with increased public awareness of the potential dangers associated with premature marketing of first-generation genomic profiles. The continued integration of personalized genomic information into the practice of cancer medicine underscores the need for a multidisciplinary model of genetic cancer risk assessment and management [Weitzel JN et al. CA Cancer J Clin 2011].
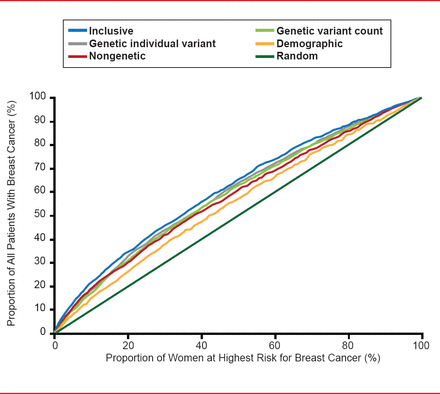
One of best examples of consolidation in this area is the Human Genome Project (Genome Wide Association Studies [GWAS]), which has identified 3 billion base pairs or 20,000 genes. The Collaborative Oncological Gene-Environment Study has brought much of the GWAS data together to look at 200,000 single-nucleotide polymorphisms in 200,000 individuals. A number of new variants (mutations) for breast, prostate, ovarian, and other cancers have been identified; however, about one half of the hereditability of cancer remains unexplained. One report suggested that when mathematically pooled, there is only modest improvement in the predicted breast cancer risk. The area under the curve (AUC) for a risk model with age, study, entry year, and four traditional risk factors was 58.0%; with the addition of 10 genetic variants, the AUC was 61.8% (Figure 1) [Wacholder S et al. N Engl J Med 2010].
Discriminatory Accuracy of Breast Cancer SNPs
Reproduced from Wacholder S et al. Performance of Common Genetic Variants in Breast-Cancer Risk Models. N Engl J Med 2010;362(11)986–993. Copyright © Massachusetts Medical Society.
With the advent of germline and tumor sequencing, “right to know” issues will have to be discussed with patients. New technology will often detect mutations that are not under review and that lack effective treatments. Patients may not want to know about these findings, but physicians have a duty to warn patients about incidental findings. Currently, the American College of Medical Genetics guidelines indicate that incidental findings must be disclosed for 24 specific conditions—16 of which are cancer syndromes.
Sequencing tumors to target therapy will mean sequencing inherited genomes to allow targeted prevention in families, and Dr. Offit believes patients must be given the choice to know or not to know.
The editors would like to thank the many members of the American Society of Clinical Oncology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2013 MD Conference Express®