Summary
This article provides an update on some key trials conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net), including important contributions from Protocol I. This study showed that anti-vascular endothelial growth factor (VEGF) therapy as an initial strategy should be the gold standard of treatment for diabetic macular edema.
- Retinal Diseases Diabetes Mellitus
- Retinal Diseases
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes Mellitus
In a symposium addressing current efforts to manage diabetic retinopathy, Lee M. Jampol, MD, Northwestern University, Chicago, Illinois, USA, provided an update on some key trials conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net), including important contributions from Protocol I. This study showed that anti-vascular endothelial growth factor (VEGF) therapy as an initial strategy should be the gold standard of treatment for diabetic macular edema (DME).
For decades, focal laser photocoagulation was the standard of care, and was highly effective, for treatment of DME. However, this technique is time-consuming and is sometimes associated with loss of central vision. In recent times, anti-VEGF agents have revolutionized the management of these conditions by targeting VEGF, an angiogenic mitogen with a pivotal role in the pathogenesis of DME.
One of the most important studies from DRCR.net is Protocol I, a randomized, controlled trial, which evaluated the efficacy of
-
intravitreal ranibizumab 0.5 mg in combination with prompt or deferred (after 6 months) laser photocoagulation,
-
prompt focal/grid laser treatment alone for treatment of central involvement DME, and
-
intravitreal triamcinolone acetonide 4 mg with prompt laser treatment.
In total, 691 patients (854 eyes) with central-involvement DME were enrolled.
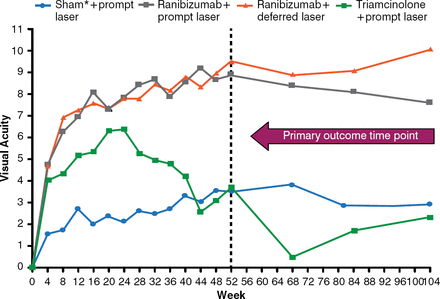
After 1 year, eyes treated with intravitreal ranibizumab and prompt or deferred laser had better visual acuity (VA) letter scores compared with focal laser with sham injection. The mean change in VA from baseline was significantly greater in the ranibizumab plus prompt laser group (p< .001) and ranibizumab plus deferred laser group (p< .001), but not in the triamcinolone plus prompt laser group (p = .31; Figure 1).
Mean Change in Visual Acuity
p values for difference in mean change in visual acuity from sham+prompt laser at the 52-week visit: ranibizumab+prompt laser < .001; ranibizumab+deferred laser < .001; and triamcinolone+prompt laser = .31.
Reproduced from Diabetic Retinopathy Clinical Research Network. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2010; 117(6):1064–1077. With permission from Elsevier.
*On November 21, 2014, this was changed from Sharm to Sham.
Visual acuity benefit often cannot be maintained in neovascular age-related macular degeneration if the frequency of ranibizumab injection is decreased from a monthly injection protocol. However, Protocol I demonstrated benefit with the use of anti-VEGF therapy for DME. Data showed that improved VA was maintained in these patients for more than 3 years of follow-up despite a decreasing number of intravitreal injections of ranibizumab: a median of 6 injections for the first 6 months, 3 injections in the second 6 months, 2 to 3 injections in the second year, and 1 to 2 injections in the third year [Diabetic Retinopathy Clinical Research Network Ophthalmology 2012; 2010].
Additional DRCR.net research in this field is underway. Protocol S and Protocol T are near completion. The noninferiority Protocol S study is comparing 2-year VA outcomes in patients with proliferative diabetic retinopathy treated with anti-VEGF therapy plus deferred panretinal photocoagulation (PRP) or standard, prompt PRP therapy. Protocol T is a comparative effectiveness study of three intravitreal anti-VEGF agents—aflibercept, bevacizumab, and ranibizumab—in patients with DME. The primary outcome is mean change in VA.
- © 2014 MD Conference Express®