Summary
Treatment of type 2 diabetes mellitus (T2DM) with saxagliptin increased the risk of major and minor hypoglycemic events, but it enabled more patients to reach an HbA1C target of less than 7% without hypoglycemia. This is according to a subanalysis of the Does Saxagliptin Reduce the Risk of Cardiovascular Events When Used Alone or Added to Other Diabetes Medications? study [SAVOR-TIMI 53; NCT01107886]. The main results of this multicenter, randomized, double-blind, placebo-controlled Phase 4 study have been published [Scirica BM et al. N Engl J Med 2013].
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus Hyperglycemia/Hypoglycemia
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Hyperglycemia/Hypoglycemia
Treatment of type 2 diabetes mellitus (T2DM) with saxagliptin increased the risk of major and minor hypoglycemic events, but it enabled more patients to reach an HbA1C target of less than 7% without hypoglycemia. This is according to a subanalysis of the Does Saxagliptin Reduce the Risk of Cardiovascular Events When Used Alone or Added to Other Diabetes Medications? study [SAVOR-TIMI 53; NCT01107886]. Itamar Raz, MD, Hadassah Medical Center, Jerusalem, Israel, presented the results. The main results of this multicenter, randomized, double-blind, placebo-controlled Phase 4 study have been published [Scirica BM et al. N Engl J Med 2013].
Hypoglycemia is a serious complication of diabetes treatment, and patients with the greatest risk of hypoglycemia need to be identified. This subanalysis aimed to identify potential predisposing factors for hypoglycemia in patients with T2DM.
The SAVOR-TIMI 53 trial randomly assigned patients with documented T2DM and established cardiovascular disease (CVD) or multiple cardiovascular (CV) risk factors to receive saxagliptin 5 mg/day (dose adjusted for reduced renal function) or placebo. The median duration of follow-up was 2.1 years, with visits every 6 months. The composite primary end points were CV death, myocardial infarction, or ischemic stroke.
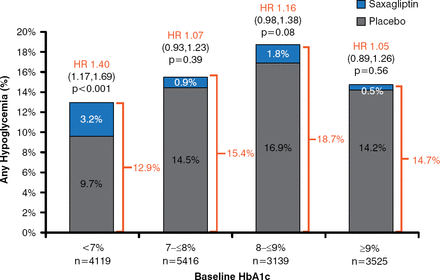
Hypoglycemia (any hypoglycemia, minor hypoglycemia, major hypoglycemia, or hypoglycemia requiring hospitalization) occurred more frequently in patients assigned to saxagliptin (Table 1). This excess risk, however, was statistically significant only in the subgroup of patients who were taking sulfonylureas at baseline. Sulfonylurea use without insulin was associated with a significantly higher rate of major hypoglycemia in the saxagliptin group versus the placebo group in patients with a baseline HbA1C level of < 7% (HR, 2.24).
Rate of Hypoglycemia by Type With Saxagliptin and Placebo
Patients in the saxagliptin arm who were taking at baseline metformin alone, insulin alone, or a sulfonylurea plus insulin or metformin, but not a sulfonylurea alone, were more likely to achieve an HbA1C < 7% without experiencing a hypoglycemic event at 1 and 2 years and at the end of the trial (Figure 1).
Effect of Saxagliptin on Hypoglycemic Events in SAVOR-TIMI 53
Reproduced with permission from I. Raz, MD.
In the overall study population, insulin, particularly short-acting insulin, was the strongest predictor for the development of hypoglycemia. In a multivariate analysis, insulin use, decreasing renal function, and increasing disease duration were all independently associated with an increased risk of hypoglycemia.
In conclusion, Prof. Raz stated that the results of this subanalysis of SAVOR-TIMI 53 indicate that insulin therapy is the strongest predictor of hypoglycemia. Treatment with saxagliptin increased the rates of major and minor hypoglycemia in the subgroups noted earlier but not the risk of hospitalization due to hypoglycemia. Major episodes of major hypoglycemia were significantly increased in those patients with a baseline HbA1C < 7% who were taking a sulfonylurea. Adding saxagliptin to conventional therapy for T2DM increased the proportion of patients whose HbA1C levels were reduced and glycemic targets achieved without hypoglycemic episodes.
- © 2014 MD Conference Express®