Summary
This article presents data from a multicenter study to compare outcomes of different formulations of estrogenic hormonal treatments in menopausal women. Results of the trial demonstrated a null effect on cardiovascular disease progression in association with hormone treatment, as well as a reduction in menopausal symptoms, and maintenance of bone mineral density.
- Menopause
- Hormone Therapy
- Endocrinology
- Diabetes & Metabolic Syndrome
- Menopause
- Hormone Therapy
Virginia Miller, MBA, PhD, Mayo Clinic, Rochester, Minnesota, USA, presented data from a multicenter study to compare outcomes of different formulations of estrogenic hormonal treatments in menopausal women. Results of the trial demonstrated a null effect on cardiovascular disease (CVD) progression in association with hormone treatment, as well as a reduction in menopausal symptoms, and maintenance of bone mineral density.
Genetic variations in estrogen metabolism and estrogen receptors account, in part, for the variability in clinical symptoms of menopause such as vasomotor instability and depression, in addition to the variability in response to exogenous hormones.
The modality of hormone delivery impacts the efficacy of the treatments because oral formulations absorbed directly into the hepatic-portal circulation have a different pharmacokinetic and pharmacodynamic profile than transdermal products, which directly enter the systemic circulation for example, oral products have a greater effect on some factors produced by the liver including low- and high-density lipoprotein cholesterol (LDL-C and HDL-C, respectively), inflammatory cytokines and proteins of the coagulation cascade [De Lignieres B et al. J Clin Endocrinol Metab 1986; Scarabin PY et al. Lancet. 2003; Bush TL et al. Circulation 1987].
Data from observational studies suggested that hormone therapy administered at menopause decreased risk factors for CVD and its adverse events [Grodstein F et al. N Engl J Med 1996; Psaty BM et al. Arch Intern Med 1994; Falkeborn M et al. Br J Obstet Gynaecol 1992; Hunt K et al. Br J Obstet Gynaecol 1990; Criqui MH et al. Am J Epidemiol 1988; Bush L et al. Circulation 1987].
The Kronos Early Estrogen Prevention Study [KEEPS; Harman SM et al. Climacteric 2005] was a multicenter, randomized, placebo-controlled, 4-year trial to compare the effectiveness of oral and transdermal estrogenic hormonal formulations (both accompanied by an oral progesterone), and placebo, in preventing progression of atherosclerosis as measured by carotid intimal medial thickness in women aged 42 to 58 years who were within 36 months of their final menstrual period. Secondary outcomes included coronary arterial calcification, blood lipids and inflammatory markers, and changes in menopausal symptoms and sexual function. Seven hundred and twenty-eight women were enrolled in the trial, and 584 completed it.
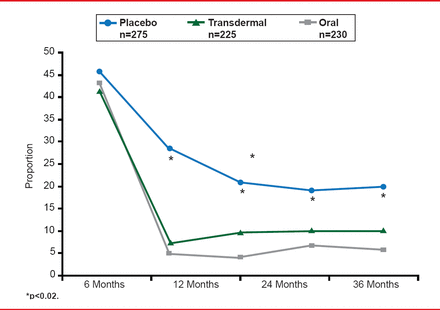
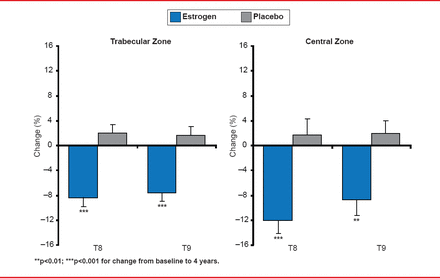
In the oral formulation group (conjugated equine estrogen 0.45 mg/day), levels of HDL-C were increased, and LDL-C were decreased, compared with those in the transdermal formulation (50 μg/day 17β-estradiol) and placebo groups. However, there was no significant effect on CVD progression. This null effect on progression of carotid intimal medial thickening, however, at least indicates that CVD was not accelerated by hormonal treatment over 4 years. Additional treatment effects in women taking estrogen/progesterone included a reduction of menopausal symptoms (Figure 1) and prevention of bone loss (Figure 2) [Farr JN et al. J Clin Endocrinol Metab 2013].
Changes in Menopausal Symptoms Throughout the KEEPS Trial
Reproduced from Farr JN et al. J Clin Endocrinol Metab 2013. With permission from The Endocrine Society.
Changes in Bone Mineral Density Throughout the KEEPS Trial
Reproduced from Farr JN et al. J Clin Endocrinol Metab 2013. With permission from The Endocrine Society.
Dr. Miller concluded by emphasizing the need for further exploration of emerging data to determine whether hormonal treatment does have a preventive effect on CVD progression over a longer time period. She also stressed the importance of avoiding generalizations about the effects of hormone treatments in this era of personalized treatments. In particular, the different types, doses, and delivery modes of hormone treatments must be carefully considered in order to maximally impact the individual health of patients.
- © 2013 MD Conference Express®