Summary
This article discusses the current landscape and future prospects in the management of acute coronary syndromes (ACS) and state of the art concepts. Specific topics include the current European Society of Cardiology algorithm to rule out non-ST-elevation myocardial infarction, results from the PROMISE trial, and a review of antithrombotic therapy for ACS.
- Interventional Techniques & Devices
- Thrombotic Disorders
- Myocardial Infarction
- Interventional Techniques & Devices
- Thrombotic Disorders
- Myocardial Infarction
- Cardiology
The current landscape and future prospects in the management of acute coronary syndromes (ACS) were addressed in a session encompassing state of the art concepts.
The current European Society of Cardiology (ESC) algorithm using clinical criteria and high-sensitivity cardiac troponin I (hs-cTnI) [Hamm CW et al. Eur Heart J 2011] correctly rules out non-ST-elevation myocardial infarction (NSTEMI) >99% of the time, said Berit Moehring, MD, University Hospital Basel, Basel, Switzerland.
For the first time, this fast track ESC algorithm incorporates measurements of hs-cTnI. It was tested in an ongoing, observational, prospective international study of consecutive patients (n=2128) presenting to the emergency department with chest pain onset <12 hours and an absence of significant ST-elevations on their electrocardiogram. The final diagnosis was adjudicated by two independent cardiologists using clinical information (pain assessment, GRACE score, and exclusion of differential diagnoses) and hs-cTnI performed on blood samples.
Patients were divided into late presenters with chest pain onset/maximum (CPM) ≥6 hours and early presenters with CPM <6 hours. In the former group, rapid rule-out was based on a single measurement using hs-cTnI, and in the latter group, on hs-cTnI values at presentation and at 3 hours. The upper limit of normal for hs-cTnI, according to the ESC algorithm, is defined as 26.2 ng/L.
Eighty-one percent (n=1729) had an hs-cTnI below the cutoff at presentation. Among the 544 late presenters, one measurement of hs-cTnI was able to rule out acute myocardial infarction (MI) in 96.0%. Twenty-two patients (1.0%) were missed by the protocol, having a final diagnosis of NSTEMI. A diagnosis of NSTEMI was ruled out in a further 19 of these 22 patients by adding clinical criteria to the hs-cTnI measurement. Therefore, with the rapid rule-out ESC protocol, 99.4% were correctly ruled out. Among the early presenters, the two hs-cTnI measurements correctly ruled out NSTEMI in 96.9%. Of the 11 patients missed using only hs-cTnI, NSTEMI was correctly ruled out in additional nine by adding the clinical criteria, meaning that a total of 99.4% were correctly ruled out.
The five missed patients have been followed for 24 months, at which time there were two MIs and one death. Pain assessment was the key additional criterion to help rule out NSTEMI, said Prof. Moehring.
EARLY ADENOSINE MAY IMPROVE STEMI OUTCOMES WITH SHORT ISCHEMIC TIMES
In patients with a first STEMI who undergo primary percutaneous coronary intervention (PCI), functional recovery and infarct mass were improved with a single early infusion of intracoronary adenosine in a subset of patients with short ischemic times. This was a major finding of the double-blind, placebo-controlled multicenter clinical trial Protection against myocardial injury in patients with ST-segment elevation [PROMISE; NCT01174550], the results of which were presented by David Garcia-Dorado, MD, Hospital Universitari Vall d'Hebron, Barcelona, Spain.
In PROMISE, 201 STEMI patients within 6 hours of symptom onset with persistent TIMI flow Grade 0/1 were randomized to receive adenosine 4.5 mg or saline given over 2 minutes distal to the lesion, immediately before PCI. Magnetic resonance imaging (MRI) was performed at 2 to 7 days and 6 months after reperfusion. The primary endpoint was infarct size by late enhancement MRI at 3 to 7 days.
There were no differences between the placebo and adenosine groups in the rates of mortality or major adverse cardiac events at 6 months. Infarct mass was not significantly different between placebo and adenosine (20.8% vs 22.5%; p=0.396), nor was relative microvascular obstruction mass (2.75% vs 2.90%; p=0.852). At 6 months, left ventricular ejection fraction (LVEF) increased significantly from baseline in patients randomized to adenosine (3.32%; p=0.006) but not placebo-treated patients (1.49%; p=0.247).
In the prespecified subgroup analysis of patients with ischemic time <200 minute, infarct mass was significantly smaller in the adenosine group (19.4% vs 25.7%; p=0.043), with no difference in microvascular obstruction mass (1.91% vs 2.94%; p=0.297) or LVEF recovery (3.6% vs 0.43%; p=0.199).
In patients with involvement of the proximal left anterior descending artery, the difference in microvascular obstruction mass (1.80% vs 5.53%; p=0.059) and improvement in LVEF (6.8%; p=0.010 vs 0.46%; p=0.897) were favorably affected by adenosine compared with placebo.
Although the results of the PROMISE trial do not support the routine use of intracoronary adenosine in STEMI patients receiving primary PCI, when considered together with previous studies [Grygier M et al. Am J Cardiol 2011; Niccoli G et al. JACC Cardiovasc Interv 2013], it does suggest that the use of adenosine in patients receiving PCI with TIMI grade flow <2 within 3 hours of symptom onset may be beneficial if confirmed, concluded Prof. Garcia-Dorado.
ANTITHROMBOTICS: THE PRESENT AND THE FUTURE
Philippe Gabriel Steg, MD, Hôpital Bichat-Claude Bernard, Paris, France, reviewed antithrombotic therapy for ACS, saying that it remains complex despite the increased flexibility allowed by new agents. In vivo, arterial thrombosis involves a confluence of platelet activation and aggregation, tissue factor generation, and fibrin formation after vascular injury [Falati S et al. Nat Med 2002]. To target this process, antiplatelet agents and anticoagulants must be combined in sequence and with overlap but the optimal cocktail of agents is not known [Hamm W et al. Eur Heart J 2011].
The future of the management of ACS will include more effective and more flexible anticoagulation, Prof. Steg predicted. Bivalirudin was associated with a reduction in 30-day mortality and non-CABG-related major bleeding when given in the hospital compared with heparin plus a glycoprotein IIb/IIIa inhibitor [EUROMAX; NCT01087723]. Drugs that work on the REG1 anticoagulation system (ie, pegnivacogin) can achieve rapid anticoagulation with injection [Dyke CK et al. Circulation 2008], but are reversible with an oligonucleotide antidote (ie, anivamersen) [Chan MY et al. J Thromb Haemost 2008]. Pegnivacogin is being compared with bivalirudin in a Phase 3 study with a planned enrollment of >13,000 patients undergoing PCI [REGULATE-PCI; NCT01848106].
Oral antiplatelet therapies have issues with long onset and long offset, which may be overcome by injectable agents, including the novel ADP-P2Y12 receptor antagonist cangrelor (plasma half-life of 5 to 9 minutes) [Bhatt DL et al. N Engl J Med 2013].
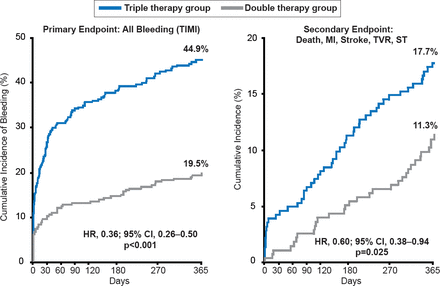
Long-term death rates post-ACS remain high [Fox KA et al. Eur Heart J 2010]. Potential therapies after discharge to improve post-acute outcomes include continuing dual antiplatelet therapy (DAPT) [Valgimigli M et al. Circulation 2012] and long-term DAPT using a more potent agent such as ticagrelor [Wallentin L et al. N Engl J Med 2009], triple therapy with an additional antiplatelet agent [Morrow DA et al. N Engl J Med 2012]; or a novel oral anticoagulant (eg, rivaroxaban) [Mega JL et al. N Engl J Med 2012]. Although the addition of rivaroxaban to aspirin and reduced the rates of cardiovascular and all-cause death, it did so at the expense of increased bleeding. Eliminating aspirin from the post-acute regimen has also been proposed on the concept that aspirin is a relatively weak antiplatelet agent and increases the risk of gastrointestinal bleeding. Aspirin added to an oral anticoagulant and clopidogrel (so called “triple therapy”) has been associated with an increase in all-cause mortality (p=0.027) and bleeding (p<0.001) compared with clopidogrel plus oral anticoagulant (Figure 1) in a large (n=496) randomized clinical trial of patients undergoing PCI [Dewilde W et al. Lancet 2013]. Reducing the intensity of antithrombotic therapy over time is another potential option [Wallentin L et al. N Engl J Med 2009].
Double Versus Triple Antithrombotic Therapy in Patients Undergoing PCI
MI=myocardial infarction; ST=stent thrombosis; TVR=target vessel revasculariztion.
Reproduced from Dewilde W et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381(9872):1107–1115. With permission from Elsevier.
- © 2013 MD Conference Express®