Summary
The nonmotor symptoms (NMS) of Parkinson's disease (PD) are common and occur across all stages of disease. Often underreported and a key determinant of quality of life, they can occur in conjunction with nonmotor fluctuations (dysautonomic, cognitive/psychiatric, and sensory/pain). NMS in PD may be associated with dopamine deficiency due to degeneration of the substantia nigra, but they may have a nondopaminergic origin as well. This article discusses several of the PD-related NMS, management strategies for patients with PD with nondopaminergic NMS, and the emerging use of animal models to improve treatment of PD-related NMS and learn about early-onset NMS.
- Mood Disorders
- Extrapyramidal & Movement Disorders
- Mood Disorders
- Neurology
- Extrapyramidal & Movement Disorders
The nonmotor symptoms (NMS) of Parkinson's disease (PD) are common and occur across all stages of disease. Often underreported and a key determinant of quality of life, they can occur in conjunction with nonmotor fluctuations (dysautonomic, cognitive/psychiatric, and sensory/pain). NMS in PD may be associated with dopamine deficiency due to degeneration of the substantia nigra, but they may have a nondopaminergic origin as well. K. Ray Chaudhuri, MD, King's College Hospital and University Hospital Lewisham, London, United Kingdom, discussed several of the PD-related NMS, including depression, constipation, pain, genitourinary problems, and sleep disorders, which can be improved with dopamine replacement therapy.
An 8-week study in patients receiving the dopamine D2 receptor agonist pramipexole reported significant baseline improvement in the Hamilton Psychiatric Rating Scale for Depression score (p<0.05), as well as the Montgomery-Asberg Depression Rating Scale and Clinician's Global Impressions-Severity of Illness scores, compared with placebo [Corrigan MH et al. Depress Anxiety 2000]. Another study suggested that depression in patients with PD might be associated with a specific loss of dopamine and noradrenaline innervation in the limbic system [Remy P et al. Brain 2005]. Current guidelines recommend pramipexole for the treatment of PD-related depression but do not recommend the dopamine agonist pergolide because of insufficient evidence to support its use in this indication.
Extended-release drug delivery might help patients with PD with NMS associated with nonmotor fluctuations. Twenty-four-hour transdermal delivery of the dopamine agonist rotigotine improved motor function control and nocturnal sleep disturbance [Trenkwalder C et al. Mov Disord 2011], as well as NMS such as fatigue, symptoms of depression, anhedonia, and apathy [Ray Chaudhuri K et al. Parkinsonism Relat Disord 2013]. Although not indicated for the treatment of depression and anxiety, IPX066 (an extended-release form of levodopa) decreases off-time in patients with PD with motor fluctuations [Hauser RA et al. Lancet Neurol 2013].
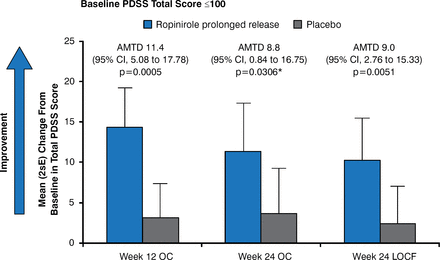
For patients with advanced PD and troublesome nocturnal disturbance not optimally controlled with levodopa, adjunctive treatment with prolonged-release ropinirole improves sleep quality, defined as an improvement on the Parkinson's Disease Sleep Scale (Figure 1) [Ray Chaudhuri K et al. Eur J Neurol 2012].
Effects of Ropinirole on Sleep Quality in Patients With PD
AMTD=adjusted mean treatment difference; LOCF=last observation carried forward; OC=observed case; PDSS=Parkinson's Disease Sleep Scale.
Reproduced with permission from John Wiley and Sons from Chaudhuri KR, Martinez-Martin P, Rolfe KA, et al. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson's disease. Eur J Neurol 2012;19:105–115.
*On November 21, 2014, this was corrected from 0.005 to 0.0306.
Prof. Chaudhuri stressed that NMS are treatable with various dopaminergic strategies, but more comparative data are needed.
Susan H. Fox, MD, PhD, University of Toronto and Toronto Western Hospital, Toronto, Ontario, Canada, followed Prof. Chaudhuri with a discussion of management strategies for patients with PD with nondopaminergic NMS such as anxiety and depression, psychosis, cognitive dysfunction, excessive daytime sleepiness, rapid eye movement (REM) sleep behavior disorder, urinary symptoms, gastrointestinal symptoms, orthostatic hypotension, and pain.
Thirty percent to 90% of patients with PD report depression. Changes in dopaminergic, noradrenergic, and serotonergic systems lead to changes in brain structure, neurotransmitter availability and function, levels of inflammation and neurotrophic factors, and stress-induced hypercortisolemia, all of which are believed to play a role in PD-related depression [Aarsland D et al. Nat Rev Neurol 2011]. Although tricyclic antidepressants appear safe in patients with PD, randomized controlled trials have produced mixed results. Despite reports of anticholinergic effects, orthostatic hypotension, and possible worsening of PD symptoms, treatment guidelines state that nortriptyline, desipramine, amitriptyline, citalopram, sertraline, paroxetine, fluoxetine, and venlafaxine may be useful treatments. Prof. Fox recommends that physicians always ask their patients about depression, be ready to use antidepressants if indicated, and confer with psychiatry colleagues when possible.
PD dementia affects attention, learning and memory, verbal fluency, visuospatial abilities, and executive functions. It is common in 30% to 80% of patients with advanced PD [Hely MA et al. Mov Disord 2008]. Cholinesterase inhibitors are often used to treat these patients, with positive effects on global assessment, cognitive function, behavioral disturbance, and activities of daily living rating scales [Rolinski M et al. Cochrane Database Syst Rev 2012]. Possible adverse events include prolonged corrected QT interval, nausea and vomiting, and worsening parkinsonism. PD dementia is also managed by reducing drug load to a minimum and the use of social support networks for long-term care. Depression or anxiety in patients with PD can be a risk for dementia.
Mild cognitive impairment affects 15% to 40% of patients with early PD. Work-related cognitive failure is a common problem in younger patients with PD (eg, difficulty multitasking jobs).
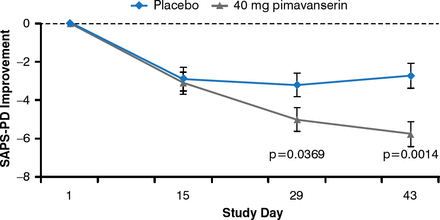
PD psychosis (illusions, sense of presence, hallucinations, paranoid delusions) are associated with dementia, mood and sleep disorders, drugs, and advancing PD disease. Clozapine appears to be an effective treatment but requires mandatory blood monitoring. In a recent randomized controlled trial, treatment with 40 mg/day of the novel 5HT2A inverse agonist pimavanserin for 6 weeks was associated with a significant (p=0.001) decrease in the PD-adapted Scale for Assessment of Positive Symptoms compared with placebo (Figure 2) [Cummings J et al. Lancet 2014].
Pimavanserin Reduces Psychosis Severity in Patients With PD
PD=Parkinson's disease; SAPS-PD=Scale for Assessment of Positive Symptoms, Adapted for Parkinson's Disease.
Reproduced with permission from Elsevier from Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 2014;383:533–540.
PD psychosis is currently best managed with clozapine and cholinesterase inhibitors to reduce visual hallucinations. Atypical antipsychotics such as olanzapine, risperidone, and aripiprazole should be avoided, as they worsen PD.
In patients with PD with autonomic failure, it is important to manage urinary and bowel function and watch for postural hypotension that can lead to falls. Drugs that lower blood pressure should be stopped immediately and salt intake increased. These patients should have small, frequent meals and avoid alcohol. Where possible, drugs for disturbances of sleep and wakefulness should be avoided, and the focus should be on sleep hygiene. Nondopaminergic pain can be managed with opioids and nonsteroidal anti-inflammatory drugs, therapies for neuropathic pain, acupuncture, and botulinum toxin. All PD-related NMS (dopaminergic and nondopaminergic) can improve with exercise.
Existing animal models offer an underused opportunity to improve treatment of PD-related NMS and learn about early-onset NMS. New models that mimic the progression of PD and possibly uncover unknown nonmotor components of PD are needed to identify additional pharmacologic targets and treatment approaches. Peter Jenner, PhD, King's College, London, United Kingdom, reviewed some of these emerging animal models.
One example of animal research in this area is the use of chronic administration of low doses of the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). In rhesus monkeys, MPTP caused attentional and executive function–type cognitive deficits, similar to those in patients with early PD [Decamp E, Schneider JS. Eur J Neurosurg 2006]. Injection of MPTP in another study with rhesus monkeys produced a dramatic disruption of sleep-wake architecture, with reduced sleep efficacy that persisted years after MPTP administration [Barraud O et al. Exp Neurol 2009]. Deregulation of REM sleep and increased daytime sleepiness occurring before the emergence of motor symptoms, and decreased dopamine turnover, were significant features of the MPTP effect.
In marmosets, MPTP causes degeneration of the cell bodies of the substantia nigra; the animals subsequently develop PD symptoms, such as bladder hyperreflexia. This suggests a model suitable for studying the mechanisms by which a loss of nigrostriatal dopamine leads to bladder hyperreflexia. Another model uses longitudinal detrusor strips from marmosets treated with MPTP [Iravani MM et al. Mov Disord 2014]. The strips from the MPTP group produced significantly greater contractions to electrical field stimulation compared with untreated animals suggesting that bladder hyperreflexivity has both a local and a central origin.
Another model using the proteasomal inhibitor MG-132 showed loss of tyrosine hydroxylase–positive cells in substantia nigra and a decrease in locomotor activity in rats up to 10 months after treatment. Neuronal loss also accrued in the locus ceruleus, raphe nuclei, and dorsal motor nucleus of the vagus, verifying that proteasomal inhibition produces a relevant model of PD [Zeng BY et al. Ann Neurol 2006]. MG-132 has the potential to damage dopaminergic elements of the substantia nigra pars compacta (SNc), however, and causes the dopaminergic neurons within the SNc to undergo degeneration, as indicated by the significant decrease in positive tyrosine hydroxylase expression, a marker for dopaminergic neurons [Wojcik S et al. Folia Neuropathol 2014].
The problem with most animal models is that they involve acute and static lesions with little applicability to premotor or NMS conditions. Models are largely based on selective loss of dopaminergic nigrostriatal pathway and do not show progression in terms of loss of dopaminergic neurons or spread of pathology as it occurs in PD. More exploration of basic automatic problems in PD, the identification of pathology responsible for NMS and the neurotransmitters involved, and the testing of pharmacologic candidates for use in humans are needed. A multidisciplinary campaign to determine the basis of nonmotor symptomatology, including clinical evaluation, imaging analysis, brain bank investigations, preclinical modeling, pharmacologic investigations, and the identification of key NMS targets (pain, sleep, depression, anxiety, autonomic dysfunction), is called for.
- © 2014 MD Conference Express®