Summary
Renal denervation is a new therapeutic intervention being studied for the treatment of resistant hypertension (HTN). The procedure has other potential applications in which it could be studied since other disease processes are characterized by increased sympathetic activity. Other renal interventions such as stenting may also relieve HTN and improve renal function, although the benefits have yet to be demonstrated. This article discusses sympathetic modulation in cardiovascular disease, including renal denervation for HTN and other potential applications, as well as multiple clinical trials.
- Interventional Techniques & Devices
- Interventional Radiology
- Hypertensive Disease
- Renal Disease
- Hypertension & Kidney Disease
- Interventional Techniques & Devices
- Cardiology & Cardiovascular Medicine
- Interventional Radiology
- Hypertensive Disease
- Renal Disease
- Hypertension & Kidney Disease
Renal denervation is a new therapeutic intervention being studied for the treatment of resistant hypertension (HTN). The procedure has other potential applications in which it could be studied since other disease processes are characterized by increased sympathetic activity. Other renal interventions such as stenting may also relieve HTN and improve renal function, although the benefits have yet to be demonstrated.
Felix Mahfoud, MD, University of Saarlandes, Homburg/Saar, Germany, discussed sympathetic modulation in cardiovascular disease, including renal denervation for HTN and other potential applications. Renal denervation has been shown to reduce systolic and diastolic blood pressure in patients with very severe treatment-resistant HTN [Krum H et al. Lancet 2013]. Reductions in blood pressure (BP) have been sustained for ≥3 years in four trials for which results have been published [Krum H et al. Lancet 2013; Hering D et al. Hypertension 2012]. Initial concerns that renal sympathetic nerves might regrow have not been realized, and functional renervation has not been detected.
Afferent renal sympathetic fibers contribute to central sympathetic activity and thereby to central sympathetic outflow when activated. The efferent fibers that connect to the heart may then increase heart rate and contractility, decrease ejection fraction, and trigger arrhythmias [Ewen S et al. Heart 2013]. In addition, afferent sympathetic fibers in the kidney can contribute to HTN by activating the renin-angiotensin system and reducing renal blood flow and glomerular filtration rate. Renal denervation reduces central sympathetic activity. Since many cardiac diseases are characterized by increased sympathetic activity, there is a growing body of evidence that renal denervation might have beneficial effects on diabetes, renal function, myocardial dysfunction, heart failure, and arrhythmias [Böhm M et al. Nat Rev Cardiol 2013].
In patients treated with renal sympathetic denervation for resistant HTN, there was a significant decrease in resting heart rate in those who had an elevated heart rate at baseline, and the reduction was greatest in patients with the highest heart rate [Ukena C et al. Int J Cardiol 2013]. Prof. Mahfoud and colleagues investigated renal denervation in a porcine model of obstructive sleep apnea (OSA) since this condition is characterized by an increase in sympathetic activity [Linz D et al. Hypertension 2012]. They found that renal denervation suppressed the BP rise which occurs following apnea. These results suggest that renal denervation could be studied in humans as a method of reducing the adverse effects of OSA. One patient with permanent atrial fibrillation (AF) showed a reduction in ventricular heart rate after renal sympathetic denervation, even during exercise. This observation led them to study the effect of renal denervation on AF. They found that they could no longer induce AF in the porcine model of OSA. A study from Russia [Pokushalov E et al. J Am Coll Cardiol 2012] randomized 27 patients with symptomatic AF and drug-resistant HTN undergoing pulmonary vein isolation (PVI) to treatment with either concomitant renal artery denervation or no additional treatment. Patients who received renal denervation in addition to PVI were significantly more likely to have no AF within the first 12 months of treatment than patients who received only PVI (69% vs 29%; p=0.033).
Prof. Mahfoud's cardiac core laboratory recently performed cardiac magnetic resonance imaging in 45 patients prior to baseline renal artery denervation and then again 6 months after treatment. They found that renal denervation used in patients with resistant HTN decreased BP and left ventricular mass [Mahfoud F, Kelle S. Unpublished data]. In the subset of patients with an left atrial enlargement, a known risk factor for developing AF, renal denervation decreased left atrial size. Renal denervation also improved circumferential strain, an early indicator of left ventricular dysfunction, by 35% (p=0.006) in patients with impaired strain at baseline. There were no significant changes in control patients who received only medical treatment.
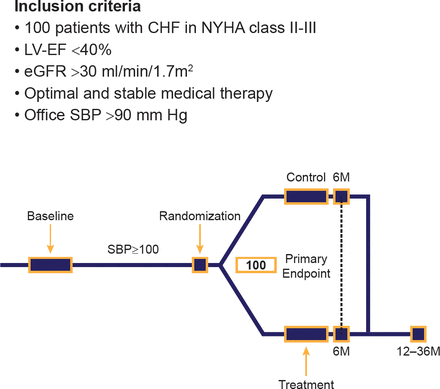
These results and the recent publication of results for the 7 initial patients in the REACH-Pilot of renal denervation for chronic heart failure trial [Davies JE et al. Int J Cardiol 2013] have provided the rationale for assessing renal denervation in chronic heart failure. The RE-ADAPT-HF trial will examine the effect of renal denervation on 100 patients with heart failure and HTN. The inclusion criteria and schema of this trial are shown in Figure 1.
RE-ADAPT-HF Trial Inclusion Criteria and Schema
CHF=congestive heart failure; eGFR=estimated glomerular filtration rate; LVEF=left ventricular ejection fraction; M=months; NYHA=New York Heart Association; SBP=systolic blood pressure.
Reproduced with permission from F Mahfoud, MD.
It is yet to be determined if renal denervation improves clinical outcomes in patients with resistant HTN. In addition, further studies are needed in order to determine if this therapy can improve outcomes of patients with chronic heart failure, obstructive sleep apnea, and chronic kidney disease.
Christopher J. Cooper, MD, University of Toledo, Toledo, Ohio, USA, presented the rationale for another trial, Benefits of Medical Therapy Plus Stenting for Renal Atherosclerotic Lesions [CORAL; Cooper CJ et al. N Engl J Med 2013], which was designed to compare medical therapy plus stenting of hemodynamically significant renal artery stenoses (RAS) versus medical therapy alone in patients with systolic HTN and RAS. Atherosclerotic RAS has been linked to secondary HTN and ischemic nephropathy. Balloon angioplasty of RAS has been performed since 1978, and data from registries suggest that stenting of atherosclerotic RAS lowers BP [Blum U et al. N Engl J Med 1997; Burket MW et al. Am Heart J 2000] and stabilizes kidney function [Harden PN et al. Lancet 1997; Watson PS et al. Circulation 2000]; however, randomized clinical outcomes trials of renal angioplasty or stenting outcomes have not yet demonstrated benefit.
The CORAL trial was an open-label, randomized, international, multicenter controlled trial sponsored by the National Heart, Lung, and Blood Institute. In order to qualify for the trial, patients were required to have HTN on ≥2 drugs, chronic kidney disease, and evidence of atherosclerosis. Patients enrolled in the trial were then randomized to either medical therapy (which included antihypertensive medications, statins, diabetes management, and smoking cessation) or renal artery stenting. The primary endpoint of the trial was a composite of death from cardiovascular or renal causes, myocardial infarction, stroke, hospitalization for heart failure, progression of renal failure, or the need for renal-replacement therapy. Medical therapy significantly reduced systolic BP in patients with chronic kidney disease with or without diabetes. The demographics of the 947 patients randomly assigned to treatment are shown in Table 1.
Baseline Demographic of the CORAL Population (n=947)
The CORAL trial has recently been completed, and data have shown that over a median follow-up period of 43 months, renal artery stenting was not better than medical management alone in preventing the primary composite endpoint (35.1% vs 35.8%; HR, 0.94; 95% CI, 0.76 to 1.17; p=0.58) [Cooper CJ et al. N Engl J Med 2013].
- © 2013 MD Conference Express®