Summary
The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction Trial [WARCEF; NCT00041938] found no compelling evidence to use warfarin for all patients according to outcomes from the study.
- Cerebrovascular Disease
- Heart Failure Clinical Trials
- Cerebrovascular Disease
- Ischemia
The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction Trial [WARCEF; NCT00041938] found no compelling evidence to use warfarin for all patients. Shunichi Homma, MD, Columbia University College of Physicians and Surgeons, New York, New York, USA, reported outcomes from the study.
WARCEF was a randomized, double-blind, multicenter, international clinical trial. The primary outcome was to determine if warfarin or aspirin was superior for preventing the combined endpoint of death, ischemic stroke, or intracerebral hemorrhage (ICH) in patients with left ventricular ejection fraction (LVEF) ≤35% in sinus rhythm. The mean follow-up was 3.5 years, ranging from 1 to 6 years.
The main secondary aim was to determine if warfarin or aspirin was superior for preventing death, ischemic stroke, or ICH plus myocardial infarction or heart failure (HF) hospitalization in patients with LVEF ≤35% in sinus rhythm.
A total of 2305 patients were randomized to receive either warfarin (target INR 2 to 3.5; n=1142) or 325 mg/day of aspirin (n=1163). Key inclusion criteria included normal sinus rhythm, LVEF ≤35%, no defined cardioembolic source, and being on an optimal HF regimen.
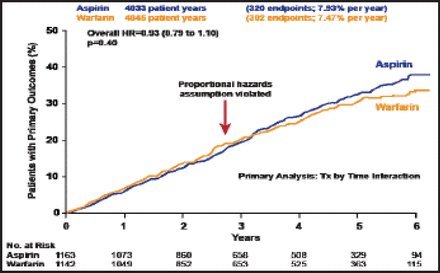
Baseline characteristics were similar between the two groups, as was baseline time in the therapeutic range (63%; 2 to 3.5). The mean INR was 2.5±0.95. The number of patient-years in the aspirin group was 4033; in the warfarin group, the number of patient years was 4045. The primary analysis was treatment-by-time interaction.
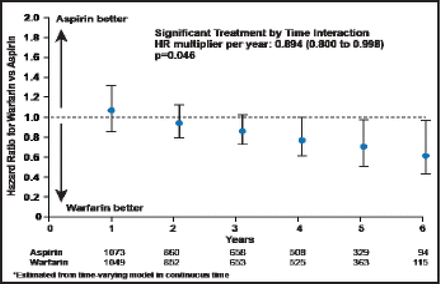
The combined primary outcome was not significantly different between groups, occurring at a rate of 7.47% per year among warfarin patients versus 7.93% per year in those who were assigned to aspirin (HR, 0.93; 0.79 to 1.10; p=0.40; Figure 1). There was, however, a suggestive benefit of warfarin for the primary outcome at 4 years and beyond (HR, 0.894; 0.800 to 0.998; p=0.046; Figure 2).
Primary Outcome.
Reproduced with permission from S. Homma, MD.
Warfarin vs Aspirin Hazard Ratios by Year of Follow-Up (Prespecified Time-Varying Analysis).
Reproduced with permission from S. Homma, MD.
The warfarin group (n=268) had a death rate of 6.63% per year. The death rate in the aspirin group (n=263) was 6.52% per year (HR, 1.01; 95% CI, 0.85 to 1.21; p=0.91). However, the difference in ischemic stroke was significant, with the per-year death rate in the warfarin group (n=23) of 0.72% compared with 1.36% in the aspirin group (n=55; p=0.005).
The main secondary outcome occurred at a rate of 12.7% per year in warfarin-treated patients versus 12.15% per year in the aspirin-treated group (p=0.33). Major hemorrhage per year, however, occurred in 1.78% of patients in the warfarin group versus 0.87% in patients who were taking aspirin (p<0.001). Significant differences were observed in gastrointestinal hemorrhage (p=0.01) and “all other bleeds” (p=0.01). Importantly, no difference in intracerebral or intracranial bleeding was found; combined, the annual rates were 0.27% in the warfarin group compared with 0.22% in the aspirin group (p=0.82).
The authors concluded that there was no overall difference for the primary outcome, although there was a suggestive benefit with warfarin at 4 years and beyond. Warfarin reduced ischemic stroke risk throughout follow-up, but patients who were on the drug had more major hemorrhages than those in the aspirin group (1.78% vs 0.87%). Intracerebral and intracranial outcomes were similar. No significant difference was observed for the main secondary outcome.
Given no overall benefit of warfarin and increased risk of bleeding, the study found no compelling evidence to use warfarin for all patients. Based on effectiveness in preventing stroke and the possible benefit of warfarin after 4 years, analyses are underway to better identify patients that will benefit from warfarin or aspirin.
- © 2012 MD Conference Express®