Summary
Outcomes from the Brazilian Intervention to Increase Evidence Usage in Acute Coronary Syndromes Trial [BRIDGE-ACS; NCT00958958] show that a simple, multifaceted, educational intervention can lead to significant improvements in the use of evidence-based medications in patients with acute coronary syndromes.
- Myocardial Infarction
- Prevention & Screening Clinical Trials
Outcomes from the Brazilian Intervention to Increase Evidence Usage in Acute Coronary Syndromes Trial [BRIDGE–ACS; NCT00958958] show that a simple, multifaceted, educational intervention can lead to significant improvements in the use of evidence–based medications in patients with acute coronary syndromes (ACS). Otavio Berwanger, MD, PhD, Research Institute Hcor–Hospital do Coração, São Paulo, Brazil, presented results from the study.
BRIDGE–ACS was a cluster–randomized (concealed allocation) trial that was conducted among 34 clusters (public hospitals) in Brazil. It enrolled a total of 1150 patients with ACS from March through November 2011, with follow–up through January 2012. The primary endpoint was the percentage of eligible patients who received all evidence–based therapies (aspirin, clopidogrel, anticoagulants, and statins) during the first 24 hours [Berwanger O et al. JAMA 2012; Berwanger O et al. Am Heart J 2012]. Secondary endpoints included adherence to all eligible evidence–based therapies during the first 24 hours and the use of aspirin, beta–blockers, statins, and ACE inhibitors at discharge; a composite evidence–based medicine score; and major cardiovascular (CV) events. CV endpoints, including mortality, CV death, recurrent ischemic events, and bleeding, were also measured as secondary endpoints. Outcomes were reviewed by blinded outcome assessors. The analyses were performed using an intention–to–treat principle.
The trial included general public hospitals from major urban areas with an emergency department that treated patients with ACS. Eligible subjects were consecutive patients who met standardized definitions of ACS (STEMI, NSTEMI, and unstable angina) as soon as they presented in the emergency department. Private hospitals, cardiology institutes, and hospitals from rural areas were excluded from the study.
The quality improvement (QI) intervention included printed reminders that were attached to the clinical evaluation form; a checklist; educational materials; an algorithm for risk stratification and recommendation of evidence–based therapies for each risk category; and color–coded bracelets according to risk stratification category.
Clusters that were randomized to the QI program received on–site training visits that were complemented by web–based and telephone training. In addition, two health professionals (a physician who acted as the local leader and a research nurse case manager) attended a workshop on how to implement the QI intervention.
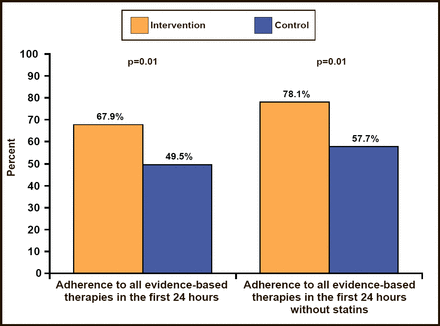
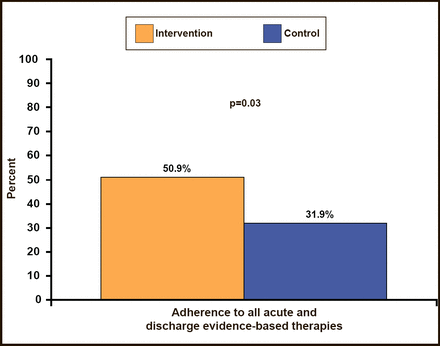
Among the 80.3% of patients who were eligible for all of the study interventions, 67.9% of those in hospitals that were randomized to the QI program received all of the evidence–based therapies in the first 24 hours versus 49.5% of patients who were randomized to hospitals without the QI program (p=0.01; Figure 1). Similarly, use of all evidence–based therapies during the first 24 hours and at discharge among eligible patients was higher in the intervention clusters versus controls (50.9% vs 31.9%; p=0.03; Figure 2). Overall, composite adherence scores were higher in QI intervention clusters than in control group clusters (89% vs 81.4%; p=0.01). There was no heterogeneity in the primary endpoint among major subgroups, including institution characteristics, such as teaching versus nonteaching, PCI availability, and cardiac surgery availability.
Adherence to All Evidence–Based Therapies in the First 24 Hours.
ZBerwanger O et al. JAMA 2012.
Adherence to All Acute and Discharge Evidence–Based Therapies.
Berwanger O et al. JAMA 2012.
Overall, the intervention had no significant difference on clinical outcomes. The rates of major CV events were 5.5% for patients from clusters that were randomized to the QI intervention versus 7.0% in control group clusters (p=0.35). There was a trend toward a reduced odds of myocardial infarction (OR, 0.25; 95% CI, 0.05 to 1.26; p=0.09) but an increase in major bleeding (OR, 6.88; 95% CI, 0.93 to 51.10; p=0.06) with intervention.
According to Dr. Berwanger, the tools that were tested in the BRIDGE–ACS trial are both simple and feasible. As such, they can become the basis for developing quality improvement programs to maximize the use of evidence–based interventions for the management of ACS.
- © 2012 MD Conference Express®