Summary
The Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial [SHIFT] was a randomized, double-blind, placebo-controlled trial in 6505 patients with moderate to severe chronic heart failure (HF), which tested whether isolated heart rate reduction with the If inhibitor ivabradine improves cardiovascular outcomes [Swedberg K et al. Lancet 2010]. Since a significant proportion of healthcare resource and economic burden is attributable to recurrent hospitalization in patients with HF, the aim of the current analysis was to assess the effect of treatment with ivabradine on recurrent hospitalizations for worsening HF throughout the entire duration of SHIFT [Borer JS et al. Eur Heart J 2012].
- Heart Failure
- Cardiology Clinical Trials
The Systolic Heart Failure Treatment with the I f Inhibitor Ivabradine Trial [SHIFT] was a randomized, double-blind, placebo-controlled trial in 6505 patients with moderate to severe chronic heart failure (HF), which tested whether isolated heart rate reduction with the I f inhibitor ivabradine improves cardiovascular (CV) outcomes [Swedberg K et al. Lancet 2010]. Inclusion criteria included hospitalization for worsening HF within 12 months prior to randomization, left ventricular ejection fraction (LVEF) ≤35%, sinus rhythm and heart rate ≥70 beats per minute (bpm), and current treatment with guidelines-based background HF therapy, including maximized β-blockade. Ivabradine was significantly better than placebo for the primary endpoint of CV death or hospitalization for worsening HF, with an 18% reduction in the cumulative frequency of events (HR, 0.82; 95% CI, 0.75 to 0.90; p<0.0001). Ivabradine versus placebo also reduced the rate of hospitalization for HF by 26% (HR, 0.74; 95% CI, 0.66 to 0.83; p<0.0001).
Since a significant proportion of healthcare resource and economic burden is attributable to recurrent hospitalization in patients with HF, the aim of the current analysis presented by Jeffrey S. Borer, MD, State University of New York Downstate Medical Center, Brooklyn and New York, New York, USA, was to assess the effect of treatment with ivabradine on recurrent hospitalizations for worsening HF throughout the entire duration of SHIFT [Borer JS et al. Eur Heart J 2012]. The endpoints were the effect of ivabradine on total heart failure hospitalizations (incidence rate ratio [IRR] vs placebo) and repeated HF hospitalizations (total-time approach: time from randomization to first, second, and third hospitalizations), as well as total CV hospitalizations and total hospitalizations for any cause. The analyses, which were post hoc, were adjusted for protocol-specified prognostic factors present prior to randomization, including β-blocker intake, New York Heart Association (NYHA) class, ischemic cause of HF, LVEF, age, systolic blood pressure (BP), heart rate, and creatinine clearance.
Prior to randomization, patients with ≥3 hospitalizations were older, had a higher heart rate, lower systolic BP, diastolic BP, and LVEF, higher NYHA class, longer duration of HF, higher incidence of diabetes, and more were taking mineralocorticoid receptor antagonists, diuretics, and digitalis, though fewer were able to tolerate β-blockers, compared with patients with <3 hospitalizations.
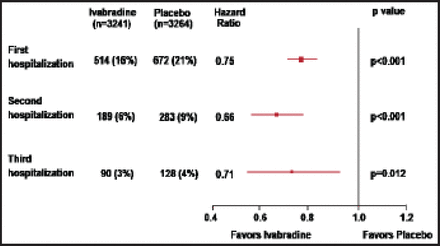
At 30 months, the cumulative incidence of HF hospitalizations was 25% lower in the ivabradine group (n=3241) versus the placebo group (n=3264). Patients in the ivabradine group versus the placebo group had significantly fewer total hospitalizations for HF (902 vs 1211; IRR, 0.75; 95% CI, 0.65% to 0.87%; p=0.0002), hospitalizations for any cause (2661 vs 3110; IRR, 0.85; 95% CI, 0.78% to 0.94%; p=0.001), and CV hospitalizations (1909 vs 2272; IRR, 0.84; 95% CI, 0.76% to 0.94%; p=0.002). Using the total-time approach, during the total follow-up interval, significantly fewer ivabradine patients versus placebo patients had a second hospitalization (6% vs 9%; HR, 0.66; 95% CI, 0.55% to 0.79%; p<0.001) and third hospitalization (3% vs 4%; HR, 0.71; 95% CI, 0.54% to 0.93%; p=0.012; Figure 1).
Recurrence of HF Hospitalization.
HF=heart failure.
Reproduced with permission from JS Borer, MD.
Heart rate reduction with ivabradine in patients with chronic HF in sinus rhythm with a heart rate ≥70 bpm and already receiving guideline-suggested therapies substantially decreased the risk of clinical deterioration as reflected by the reduction in total hospitalizations for worsening HF, reduction in the incidence of recurrent HF hospitalizations, and increase in time to first and subsequent hospitalizations. This benefit reduces the total burden of HF for the patient and can be expected to substantially reduce healthcare costs. These findings are consistent with the 2012 European Society of Cardiolgy heart failure guidelines that recommend ivabradine for the reduction of HF hospitalization in patients who meet the SHIFT trial's eligibility criteria, and who are treated with maximal HF therapy, including an ACEI or ARB, maximized β-blockade, and mineralocorticoid receptor antagonist.
- © 2012 MD Conference Express®