Summary
This article gives an overview of the key messages related to cardiovascular disease (CVD) prevention in the the Fifth Joint Task Force of the Guidelines on CVD prevention in clinical practice [Perk J et al. Eur Heart J 2012]. Therapeutic guideline updates include acute and chronic heart failure, acute myocardial infarction with ST-segment elevation, atrial fibrillation, valvular heart disease, an an updated third universal definition of myocardial infarction.
- Arrhythmias
- Myocardial Infarction
- Cardiology Guidelines
- Featured Meeting - Specialty page
- Heart Failure
- Valvular Disease
Guidelines Overview
Joep Perk, MD, Linnaeus University, Kalmar, Sweden, gave an overview of the key messages related to cardiovascular disease (CVD) prevention in the the Fifth Joint Task Force of the Guidelines on CVD prevention in clinical practice [Perk J et al. Eur Heart J 2012]. Over 50% of CVD mortality reductions are due to changes in risk factors, while 40% are related to improved treatments. Among the new concepts are 4 levels of CVD risk (very high, high, moderate, low), risk-factor screening for men >40 years and women >50 years or if postmenopausal, risk-age concept, importance of psychosocial risk factors, limited role of novel risk biomarkers; no exposure to passive smoking, role of specific diet patterns, and multimodal behavioral intervention effectiveness. Table 1 outlines the key recommendations for blood pressure, diabetes, and lipids.
Key BP, Diabetes, and Lipid Recommendations.
Acute and Chronic Heart Failure
John McMurray, MD, Glasgow University, Glasgow, United Kingdom, summarized the new 2012 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure (HF) [McMurray JJ et al. Eur J Heart Fail 2012]. The main changes from 2008 for the treatment of chronic HF are expanded indications for mineralocorticoid receptor antagonists (MRAs), a new indication for the sinus node inhibitor, ivabradine, an expanded indication for cardiac resynchronization therapy (CRT), new information on coronary revascularization in systolic HF, recognition of the growing use of ventricular assist devices, and the emergence of transcatheter valve interventions. New diagnostic tools for HF include mid-regional pro-atrial natriuretic peptide, 3D and strain imaging echocardiography, computed tomography coronary angiography, cardiac magnetic resonance, and genetic testing.
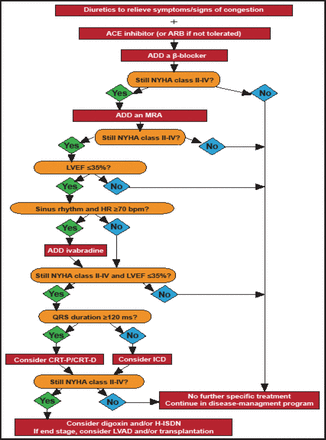
Treatment options for chronic symptomatic systolic HF are shown in Figure 1. Treatment should begin with diuretics plus an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) with the addition of a β-blocker in patients with left ventricular ejection fraction (LVEF) ≤40%. Adding an MRA is recommended followed by ivabradine if the patient remains symptomatic, with an LEVF ≤35%, and is in sinus rhythm with a heart rate ≥70 beats per minute (bpm). An implantable cardioverter-defibrillator should be considered in patients with persistent NYHA Class II and III and LVEF ≤35%; for those who also have a QRS duration ≥120 ms, CRT should be considered. An LV assist device or biventricular assist device is recommended in select patients with end-stage HF.
Treatment Options for Patients with Chronic Symptomatic Systolic HF.
ACE=agiotensin-converting enzyme; ARB=angiotensin receptor blocker; CRT-D=cardiac resynchronization therapy defibrillator; CRT-P=CRT plus pacemaker; H-ISDN=hydralazine and isosorbide dinitrate; HR=heart rate; ICD=implantable cardioverter-defibrillator; LVAD=left ventricular assist device; LVEF=left ventricular ejection fraction; MRA=mineralocorticoid receptor antagonist.
Reproduced with permission from the European Society of Cardiology. All rights reserved. Copyright © 2012.
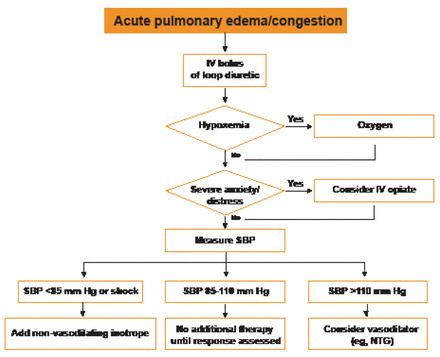
The new algorithm for management of acute HF is shown in Figure 2.
Initial Assessment of a Patient with Suspected HF.
IV=intravenous; NTG=nitroglycerin; SBP=systolic blood pressure.
Reproduced with permission from the European Society of Cardiology. All rights reserved. Copyright © 2012.
Acute Myocardial Infarction with ST-Segment Elevation
Philippe Gabriel Steg, MD, Hôpital Bichat, Paris, France, and Stefan James, MD, PhD, Uppsala University Hospital, Uppsala, Sweden, presented the changes and additions to the new European Society of Cardiology (ESC) Guidelines on ST-segment elevation myocardial infarction (STEMI), which include expanded sections on early diagnosis and cardiac arrest as well as updated recommendations on pre-hospital logistics of care, reperfusion strategies, percutaneous coronary intervention (PCI) strategies, and routine therapies [Steg PG et al. Eur Heart J 2012].
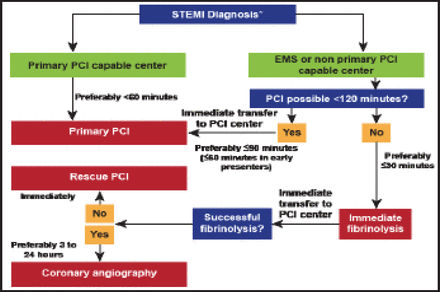
In the emergency setting, an ECG should be recorded within 10 minutes of first medical contact (FMC). Reperfusion is indicated in all patients with symptoms of <12 hours and persistent STE or new left bundle branch block, and in patients with ongoing ischemia even if symptoms started >12 hours before. For patients presenting with a STEMI to a PCI-capable hospital, the guidelines advise primary PCI, to be achieved within <60 minutes (Figure 3). For patients with STEMI presenting to a non-PCI-capable hospital, the selection of reperfusion therapy is based on whether PCI is possible in ≤120 minutes; if it is, PCI should be attempted with a target of FMC to primary PCI, ≤90 minutes. If not, patients should receive immediate fibrinolysis with a target time from FMC to fibrinolysis, ≤30 minutes. Following successful fibrinolysis, routine angiography is recommended within a window of 3 to 24 hours.
Prehospital and In-Hospital Management and Reperfusion Strategies within 24 Hours of FMC.
*The time point the diagnosis is confirmed with patient history and ECG ideally within 10 minutes from the FMC; All delays are related to FMC; ECG=electrocardiogram; EMS=emergency medical service; FMC=first medical contact; PCI=percutaneous coronary intervention; STEMI= ST-segment elevation myocardial infarction.
Reproduced with permission from the European Society of Cardiology. All rights reserved. Copyright © 2012.
Other key recommendations include the following:
-
Primary PCI with stenting is preferred over thrombolysis
-
Limit primary PCI to culprit vessel
-
Radial access preferred over femoral access if operator experienced
-
Drug-eluting stent preferred to bare-metal stent if no prolonged dual antiplatelet therapy contraindications
-
Aspirin antiplatelet therapy, with the addition of an adenosine diphosphate–receptor blocker
-
Consider Gb IIb/IIIa inhibitors for bailout therapy
-
Injectable anticoagulant required for primary PCI; bivalirudin preferred and enoxaparin may be preferred over unfractionated heparin
Atrial Fibrillation
John Camm, MD, St. George's University, London, United Kingdom, presented the 2012 focused update on the ESC Guidelines for the management of atrial fibrillation (AF) [Camm AJ et al. Eur Heart J 2012]. The focus of the 2012 update was on anticoagulation risk stratification, novel oral anticoagulants (NOACs), left atrial appendage (LAA) occlusion/excision, pharmacologic cardioversion, oral antiarrhythmic therapy, and left atrial catheter ablation.
Antithrombotic therapy is recommended for all patients with AF except those at low risk or with contraindications. OAC therapy is recommended in patients with CHA2DS2-VASc score ≥2 and should be considered in those with CHA2DS2-VASc score of 1, with an adjusted-dose vitamin K antagonist (VKA), direct thrombin inhibitor, or factor Xa inhibitor. If patients refuse any OAC, antiplatelet therapy should be considered. A NOAC is recommended when an adjusted-dose VKA cannot be used. The oral antiarrhythmic dronedarone is recommended for patients with recurrent AF but not in patients with permanent AF due to an increase in mortality in the latter group.
LAA closure may be considered in patients with high stroke risk and contraindications for long-term OAC. When pharmacologic cardioversion is preferred and there is no or minimal structural heart disease, IV flecainide, propafenone, ibutilide, or vernakalant are recommended. Catheter ablation is recommended in patients who have symptomatic recurrences of AF on antiarrhythmic drug therapy and who prefer further rhythm control therapy.
Valvular Heart Disease
The 2012 ESC/European Association for Cardio-Thoracic Surgery (EACTS) Guidelines on the management of valvular heart disease were developed because of new evidence on risk stratification, diagnostic methods, and therapeutic options [Vahanian A et al. Eur Heart J 2012; Eur J Cardio Thorac Surg 2012]. Alec Vahanian, MD, Hôpital Bichat, Paris, France, and Ottavio Alfieri, MD, Università Vita-Salute San Raffaele, Brescia, Italy, presented an overview of these updates. The guidelines address the following key areas: patient evaluation, aortic regurgitation (AR), aortic stenosis (AS), mitral regurgitation (MR), tricuspid disease, and valve prostheses.
Patients should be evaluated for symptoms, severity of valvular disease, life expectancy, quality of life, and benefits versus risks of intervention. In the absence of a perfect quantitative score, risk assessment should primarily rely on the heart team's clinical judgment in addition to a combination of scores.
Surgery is recommended for patients with AR with a significantly enlarged ascending aorta, severe symptomatic AR, or severe asymptomatic AR with LVEF ≤50% or LV end-diastolic diameter ≥70 mm or LV end-systolic diameter >50 mm (or >25 mm/mm2 body surface area). Transcatheter aortic valve implantation (TAVI) is indicated for patients with severe symptomatic AS not suitable for surgical aortic valve replacement (SAVR) with a life expectancy of >1 year.
TAVI should not be performed in patients at intermediate risk for surgery. SAVR is indicated for the following:
-
Severe symptomatic AS
-
Patients undergoing coronary artery bypass graft (CABG) surgery, ascending aorta surgery, or other valve surgery
-
Severe asymptomatic AS with systolic LV dysfunction, abnormal exercise test showing AS-related symptoms, or if low surgery risk and peak transvalvular velocity >5.5 m/s or severe valve calcification and peak transvalvular velocity progression ≥0.3 m/s per year
Mitral valve repair for symptomatic severe primary MR is preferred when it is expected to be durable. For secondary severe MR, surgery is indicated in patients undergoing CABG and who have LVEF >30% and should be considered for patients with LVEF <30%, option for revascularization, and evidence of viability.
Third Universal Definition of Myocardial Infarction
Joseph S. Alpert, MD, University of Arizona College of Medicine, Tucson, Arizona, USA, presented the ESC third universal definition of MI [Thygesen K et al. Eur Heart J 2012]. Table 2 shows the definitions of the 5 MI classifications.
Universal Classification of MI.
- © 2012 MD Conference Express®