Summary
This article reviews the latest advances in carotid stenting for the management of stroke, including carotid stenting for prevention of ipsilateral stroke, carotid endarterectomy versus carotid artery stenting, and embolic protection and patient selection.
- Interventional Techniques & Devices
- Cerebrovascular Disease
- Valvular Disease
- Cerebrovascular Disease
- Interventional Techniques & Devices
Carotid Stenting for Prevention of Ipsilateral Stroke
Internal carotid artery stenosis accounts for 20% of all ischemic strokes. Carotid artery stenosis is considered symptomatic in the presence of transient ischemic attack (TIA) or stroke affecting the corresponding territory within the previous 6 months. The North American Symptomatic Carotid Endarterectomy Trial [NASCET] found that the risk of recurrent ipsilateral stroke in patients with symptomatic carotid artery stenosis treated conservatively was 4.4% per year for patients with 50% to 69% stenosis and 13% per year for those with >70% stenosis. The risk of recurrent TIA or stroke is 10% to 30% in the first month [Tendera M et al. Eur Heart J 2011].
Prof. Habib Gamra, MD, Fattouma Bourguiba University Hospital, Monastir, Tunisia, reviewed the latest advances in carotid stenting for the management of stroke. Accurate assessment and revascularization should be undertaken very early after a TIA. The decision to revascularize is based on the presence of signs or symptoms related to the affected carotid artery; the degree of internal carotid artery stenosis; and other factors including patient age, gender, comorbidities, and life expectancy [Tendera M et al. Eur Heart J 2011].
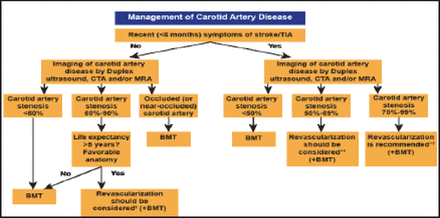
Patients with carotid artery disease may present with hemispheric ischemia, which manifests as a combination of weakness, paralysis, numbness, and/or tingling on the side of the body contralateral to the culprit artery. Another possible clinical manifestation is temporary or permanent partial or total blindness in the ipsilateral eye, caused by emboli to the retinal artery. Duplex ultrasound, computed tomography angiography, and/or magnetic resonance angiography are recommended by the European Society of Cardiology (ESC) for evaluation of carotid artery stenosis and to determine the need for revascularization (Figure 1) [Tendera M et al. Eur Heart J 2011].
ESC Guidelines for Management of Carotid Artery Disease.
*The management of symptomatic carotid artery disease should be decided as soon as possible (<14 days after onset of symptoms); †After multidisciplinary discussion including neurologists; BMT=best medical therapy; CTA=computed tomographic angiography; MRA=magnetic resonance angiography; TIA=transient ischemic attack.
Reproduced with permission from The European Society of Cardiology.
Carotid Endarterectomy Versus Carotid Artery Stenting
Carotid endarterectomy (CEA) was shown to be more effective than medical management for the endpoint of stroke and death rate in NASCET (5.8%) and the Asymptomatic Carotid Atherosclerosis Study [ACAS] (2.7%). Carotid artery stenting (CAS) is less invasive than CEA, is performed under local anesthesia, avoids the risk of peripheral nerve damage associated with neck dissection, and is less painful. The goal of CAS is to lower the risk of ipsilateral carotid, plaque-related stroke.
Several studies have compared CAS with CEA (Table 1). The only current head-to-head trials of CAS versus CEA are the Carotid and Vertebral Artery Transluminal Angioplasty Study [CAVATAS; CAVATAS Investigators. Lancet 2001], Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy [SAPPHIRE; Gurm HS et al. N Engl J Med 2008], and Carotid Revascularization Using Endarterectomy or Stenting Systems [CaRESS; Di Mario C et al. Lancet 2008] studies. The 30-day data showed no significant difference in stroke or death rates between CAS and CEA in the CAVATAS (10% vs 10%), SAPPHIRE (4.8% vs 5.6%), and CaRESS studies (2.1% vs 3.6%; Table 2). For the endpoint of stroke, death, or myocardial infarction (MI) with CAS vs CEA, there was no significant difference in the CAVATAS (10% vs 11%), CaRESS (2.1% vs 4.4%), and SAPPHIRE (4.8% vs 9.8%) trials, although the difference was close to reaching significance in the SAPPHIRE study (p=0.06).
Studies of CAS Versus CEA.
30-Day Results: CAS Versus CEA.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis [EVA-3S] trial randomized 527 symptomatic patients with stenosis ≥60% to CAS or CEA, with a primary endpoint of the cumulative incidence of any stroke or death within 30 days of treatment [Mas JL et al. N Engl J Med 2006]. The trial was stopped early because of significantly increased event rates in the CAS arm (9.6%) versus the CEA arm (3.9%; RR, 2.5; 95% CI, 1.2 to 5.1; p=0.01).
The Carotid Revascularization Endarterectomy Versus Stenting Trial [CREST] randomized 2502 asymptomatic and symptomatic patients to CAS versus CEA [Brott TG et al. N Engl J Med 2010]. There was no difference in the primary endpoint of periprocedural stroke, MI, or death, plus ipsilateral stroke at 4 years between CAS (7.2%) and CEA (6.8%; HR for CAS, 1.11; 95% CI, 0.81 to 1.51; p=0.51). However, CAS was more effective than CEA in patients <70 years (p=0.02). Twice as many acute MIs occurred in the CEA group (2.3%) versus the CAS group (1.1%; p=0.03). The difference in overall stroke rate was 4.1% with CAS versus 2.3% with CEA (p=0.01) but there was no difference in major disabling strokes between the 2 groups.
A meta-analysis of 13 randomized trials (n=7484) found that CAS versus CEA was associated with an increased risk of any stroke (RR, 1.45; 95% CI, 1.06 to 1.99), decreased risk of periprocedural MI (RR, 0.43; 95% CI, 0.26 to 0.71), and a nonsignificant increase in mortality (RR, 1.40; 95% CI, 0.85 to 2.33) [Economopoulos KP et al. Stroke 2011; Tendera M et al. Eur Heart J 2011].
Embolic Protection and Patient Selection
Embolic protection devices (EPDs) are an accepted part of CAS designed to reduce the risk of periprocedural stroke. Several types exist, including temporary occlusion and aspiration devices, filter devices, and flow reversal devices.
In the EVA-3S trial, CAS without an EPD was halted because of excessive stroke risk compared with EPD use (OR, 3.9; 95% CI, 0.9 to 16.7) [Tendera M et al. Eur Heart J 2011]. The ESC Guidelines on the diagnosis and treatment of peripheral artery diseases recommend dual antiplatelet therapy with aspirin and clopidogrel for patients undergoing CAS, and the use of EPDs may be considered.
Selection of patients for CAS is important. High-risk patients include asymptomatic patients >80 years of age, patients with access problems, patients with a large neurologic defect at baseline, patients with marked cerebral atrophy and microangiopathy, and those with dementia. High-risk lesions include those with obvious filling defect or thrombus; vessel occlusion; severe distal loops, kinks, or bends; and heavy concentric calcifications.
The ESC Guidelines say CEA should be considered in asymptomatic patients with carotid artery stenosis ≥60% if the perioperative stroke and death rate for procedures performed by the surgical team is <3% and the patient's life expectancy exceeds 5 years [Tendera M et al. Eur Heart J 2011]. CAS may be considered as an alternative in high-volume centers with a documented death or stroke rate <3%. In symptomatic patients, CEA is recommended for those with 70% to 99% stenosis and should be considered for those with 50% to 69% stenosis, depending on patient-specific factors. For symptomatic patients at high surgical risk requiring revascularization, the guidelines recommend CAS as an alternative to CEA. In symptomatic patients requiring revascularization, CAS may be considered as an alternative to CEA in high-volume centers with a documented death or stroke rate of <6%.
Carotid stenting is an appealing noninvasive procedure with great potential, particularly in the management of stroke. However, the indications for carotid stenting remain limited and patient selection is crucial. Operator expertise is even more important and has been shown to influence outcomes.
- © 2012 MD Conference Express®