Summary
Significant advancements in the knowledge of human genetics and type 2 diabetes (T2DM) have been made in the last 5 years. Researchers have now identified ∼65 regions of the genome that influence diabetes [Morris AP et al. Nat Genet 2012]. Genetic studies have also become larger. These advances are virtually all tied to improvements in technology that now allow researchers to quickly sequence the genomes of thousands of individual patients and to analyze tens of millions of genetic variants. This article discussed the new biology being derived from genetic research and how it can impact research being conducted in nongenetic areas.
- Diabetes Mellitus
Significant advancements in the knowledge of human genetics and type 2 diabetes (T2DM) have been made in the last 5 years. It was only in 2007 that one of the first genome-wide association studies (GWAS) for T2DM identified the first genetic polymorphism with a robust relationship to diabetes—a common variant in the fat mass and obesity associated FTO gene that predisposes individuals to diabetes through an effect on body mass index (BMI) [Frayling TM et al. Science 2007]. Researchers have now identified ∼65 regions of the genome that influence diabetes [Morris AP et al. Nat Genet 2012]. Genetic studies have also become larger. The 2007 study included ∼39,000 participants, while current studies of BMI include up to 350,000. These advances are virtually all tied to improvements in technology that now allow researchers to quickly sequence the genomes of thousands of individual patients and to analyze tens of millions of genetic variants.
Timothy Frayling, PhD, University of Exeter, Exeter, United Kingdom, discussed the new biology being derived from genetic research and how it can impact research being conducted in nongenetic areas.
Most forms of diabetes do not follow strict patterns of inheritance through families; they tend to appear in clusters. Prof. Frayling's approach to genetic studies focuses on differences in allele frequency based on the principle that variant genes present more frequently in cases than in controls, thus variant status provides probability of disease status. However, he cautioned that it remains extremely difficult to identify a single causal risk factor to explain the huge variation in human beings and to sort out the effects of confounding factors.
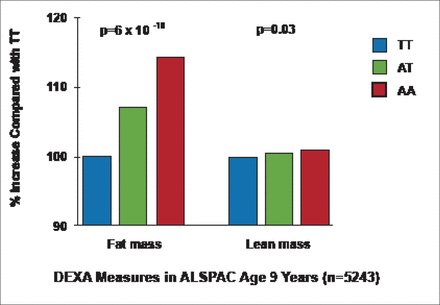
Geneticists have identified 32 polymorphisms that are robustly associated with normal variation in BMI, some of which overlap with the monogenic causes of severe obesity. Some of the common polymorphisms are located near genes such as POMC, BDNF, SH2B1, and MC4R—mutations known to cause severe appetite disorders and severe obesity in children [Speliotes EK et al. Nat Genet 2010]. However, the FTO genotype still stands out as the having the largest influence on BMI and related metabolic traits, and dual-energy X-ray absorptiometry measures in children participating in the 2007 study [Frayling TM et al. Science 2007] show that the FTO effect is associated entirely with adiposity, as opposed to skeletal or lean tissue mass (Figure 1).
FTO: The Association is with Fat Mass Rather Than Lean Mass.
ALSPAC=Avon Longitudinal Study of Parents and Children; DEXA=dual-energy X-ray absorptiometry.
Reproduced with permission from TM Frayling, PhD.
Longitudinal studies have also informed today's knowledge of how the FTO gene functions. Sovio et al. [PloS Genet 2011] have shown that the minor (fat) allele in FTO is associated with children emerging from their adiposity trough earlier than the major (thin) allele by the age of 7 years. Thus, after the age of 7 it is difficult to assess the effects of FTO gene variants in humans because fatter individuals eat, behave, and metabolize differently than thinner individuals as a consequence of being more overweight. This association has also been documented in an animal study in which enhanced expression of FTO led to increased food intake and obesity [Church C et al. Nat Genet 2010].
Interactions between the FTO gene and the environment have been the subject of several studies. It was recently shown that the FTO genotype influences individual variation in BMI as well as mean BMI [Yang J et al. Nature 2012]. This concept, which indicates a possible increase in the strength of genetics in today's environment, was also seen in the results of a twin study that showed strong evidence that adiposity in preadolescent children born since the onset of the obesity epidemic is highly heritable, while environmental effects are small and divided approximately equally between shared and nonshared effects [Wardle J et al. Am J Clin Nutr 2008]. After categorizing subjects as physically inactive or active, results from a meta-analysis of data from 45 studies of adults (n=218,166) and 9 studies of children and adolescents (n=19,268) showed FTO had a weaker effect on the distribution of BMI in physically active individuals, suggesting that the genetic effects in a less obesogenic environment are stronger [Kilpeläinen TO et al. PLoS Med 2011]. Another impact study focused on the interaction of sugar-sweetened beverages with the genetic predisposition to adiposity. The genetic association with BMI and adiposity was stronger among participants with higher intake of sugar-sweetened beverages than among those with lower intake [Qi Q et al. N Engl J Med 2012]. “There is still much to learn about the FTO gene, but the pieces are starting to come together,” said Prof. Frayling.
Genetic studies are also informing epidemiology. Since genes are randomly sorted during meiosis, genetic studies are analogous to a randomized controlled trial. It is generally accepted that testosterone levels in men are inversely associated with several recognized risk factors for T2DM (eg, obesity, central adiposity, and elevated levels of fasting plasma insulin and glucose). A relationship has also been indicated between T2DM and lower baseline levels of free testosterone and sex hormone-binding globulin (SHBG) in men [Tibblin G et al. Diabetes 1996; Haffner SM et al. Am J Epidemiol 1996] and reduced SHBG concentrations among women with polycystic ovary syndrome and hyperinsulinemia [Nestler JE et al. J Clin Endocrinol 1991; Stellato RK et al. Diabetes Care 2000]. Using Mendelian randomization principles, Prof. Frayling's laboratory has shown that GWAS have shown genetic variants at the SHBG gene influence circulating SHBG levels and that there is a direct relationship between low SHBG levels and increased risk of T2DM. Prof. Frayling predicted that future Mendelian randomization experiments will be useful for predicting which epigenetic and gene expression factors casually influence T2DM.
Increased adiponectin levels have been shown to be associated with a lower risk of T2DM. Thus, the relationship between genetic variation at the adiponectin-encoding gene, ADIPOQ, and adiponectin levels, and subsequently its role in diabetes has been studied extensively. One study identified a novel association between a low-frequency single nucleotide polymorphism (SNP; rs17366653) and adiponectin levels, and showed that 7 SNPs exert independent effects on adiponectin levels, which explained 6% of adiponectin variation. No evidence of association with T2DM was found [Warren LL et al. Diabetes 2012]. Large-scale and well-powered Mendelian randomization is recommended for future studies.
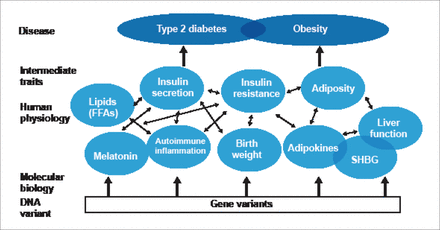
Prof. Frayling concluded with a description of an ongoing study comparing ADIPOQ gene splicing, metabolic profiles, and insulin resistance in individuals with lifelong genetically reduced adiponectin levels with a control population. Data for this study and others are and will be available on the Internet. Prof. Frayling reiterated that GWAS findings are important tools to understand the biology of diabetes and should be made freely available to all researchers (Figure 2).
Main Message: GWAS Findings Are Important Tools to Understand the Biology of Diabetes.
DNA=deoxyribonucleic acid; FFA=free fatty acid; SHBG=sex hormone-binding globulin.
Reproduced with permission from TM Frayling, PhD.
- © 2012 MD Conference Express®