Summary
In 2011, 366 million people had diabetes; by 2030, that figure is projected to rise to 552 million [International Diabetes Federation. IDF Diabetes Atlas. 5th ed. 2009]. The search for novel oral antidiabetic drugs has taken on a growing sense of urgency. This article discusses efforts underway to develop glucagon-based incretin hybrids.
- Diabetes Mellitus
- Insulin Diabetes & Metabolic Syndrome
In 2011, 366 million people had diabetes; by 2030, that figure is projected to rise to 552 million [International Diabetes Federation. IDF Diabetes Atlas. 5th ed. 2009]. The search for novel oral antidiabetic drugs (OADs) has taken on a growing sense of urgency. Richard D. DiMarchi, PhD, Indiana University, Bloomington, Indiana, USA, discussed efforts underway to develop glucagon-based incretin hybrids.
Dr. DiMarchi focused his discussion on 2 glucagon-based single molecule coagonists: glucagon/glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP)/GLP-1. The clinical benefits of each are shown in Table 1. The glucagon/GLP-1 coagonist hypothesis is that chronic glucagon action decreases fat mass by increasing energy expenditure via the glucagon receptor, GLP-1 decreases fat mass by reducing food intake via the GLP-1 receptor, a GLP-1/glucagon coagonist might decrease fat mass by synergistically affecting both components via 2 receptors, and a GLP-1/glucagon coagonist should minimize the diabetogenic risk of a pure glucagon analogue.
Summary of Glucagon-Based Single Molecule Coagonists.
Several parts of the hypothesis have been proven in preclinical and clinical studies. Day et al. [Nat Chem Biol 2009] reported a new peptide with agonism at the glucagon and GLP-1 receptors that has potent, sustained satiation-inducing and lipolytic effects. Two coagonist peptides that differ from each other only in their levels of glucagon receptor agonism were studied in rodent obesity models. Administration of PEGylated peptides once per week normalized adiposity and glucose tolerance in diet-induced obese (DIO) mice.
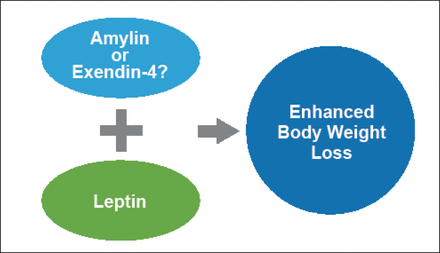
Preclinical evidence also indicates that high-activity, long-acting leptin analogues are additively efficacious when used with other weight-lowering agents, ie, extendin-4 or fibroblast growth factor 21 (FGF21; Figure 1) [Muller TD et al. J Pept Sci 2012].
High-Activity, Long-Acting Leptin Analogues May Be Additively Efficacious When Used with Other Weight-Lowering Agents.
Reproduced with permission from RD DiMarchi, PhD.
While Schelshorn et al. [Mol Pharmacol 2012] demonstrated that GLP-1 induces G-protein-coupled receptor heteromer formation, Christensen et al. [Diabetes 2011] found that GIP appears to be a physiological bifunctional blood glucose stabilizer with diverging glucose-dependent effects on the 2 main pancreatic glucoregulatory hormones in healthy human subjects.
Based on the data, Dr. DiMarchi concluded that GLP-1 agonists provide significant clinical benefits, yet glucagon/GLP-1 and GIP/GLP-1 coagonists deliver significantly greater activity than GLP-1 in animals. The addition of leptin provides additional efficacy in DIO mice.
Targeting the Glucocorticoid Pathway
André J. Scheen, MD, PhD, University of Liège, Liège, Belgium, addressed the question of whether there is any role of cortisol in the prevention of hyperglycemia in type 2 diabetes mellitus (T2DM). He discussed similarities between T2DM, metabolic syndrome, and Cushing syndrome; the role of cortisol on activation of the hypothalamic-pituitary-adrenal axis and local tissue regulation; the role of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in adipose tissue and the liver; the effects of 11β-HSD1 inhibition (knockout models and chemical inhibitors) in rodents; the effects of selective 11β-HSD1 inhibitors in humans with T2DM (and metabolic syndrome); and limitations and perspectives.
Hollis and Huber [Diabetes Obes Metab 2011] reported that 11β-HSD1 catalyzes the intracellular conversion of inert cortisone to physiologically active cortisol, enhancing local cortisol action beyond what would be predicted based on simple plasma exposures.
Results of a 12-week, placebo-controlled dose-ranging efficacy study by Rosenstock et al. [Diabetes Care 2010] provided the first evidence that decreasing local cortisol exposure through selective 11β-HSD1 inhibition can improve hyperglycemia in T2DM. Treatment with INCB13739 showed statistically significant reductions in HbA1C in the 100-mg (−0.47%; p<0.05) and 200-mg (−0.56%; p<0.01) groups. The 200-mg group also achieved significant reductions relative to placebo in fasting plasma glucose (−24 mg/dL) and homeostasis model assessment-insulin resistance (−24%).
In obese men with T2DM, liver 11β-HSD1 is increased, whereas liver 11β-HSD1 is sustained in obese euglycemic men. This supports the concept that inhibitors of 11β-HSD1 are likely to be most effective in obese T2DM subjects [Stimson RH et al. Diabetes 2011].
In addition to 11β-HSD1 inhibitors, which reduce the glucocorticoid effects in liver and fat, other novel approaches to glycemic regulation include the use of sodium-glucose cotransporter 2 inhibitors, which increase renal glucose elimination. Insulin glucokinase activators and pancreatic-G-protein-coupled fatty-acid receptor agonists, glucagon receptor antagonists, and metabolic inhibitors of hepatic glucose output are being assessed. Early proof of principle has been shown for compounds that enhance and partly mimic insulin action and replicate some effects of bariatric surgery [Tahrani AA et al. Lancet 2011].
Enhancing the Action of Insulin
Stefano Del Prato, MD, University of Pisa, Pisa, Italy, discussed new routes to enhancing the action of insulin.
Ma et al. [Mol Cell Biochem 2011] have investigated the effects of compound CCF06240, a PTP1B inhibitor, on insulin sensitivity and lipid abnormalities in vivo and in vitro. PTP1B is a negative regulator of the insulin signaling pathway. Results demonstrate that CCF06240 could increase insulin sensitivity through the regulation of the insulin signaling pathway, and decrease free fatty acid-insulin-induced hepatocytes lipid accumulation by reducing fatty acid synthesis.
Conti et al. [Diabetes 2011] developed teglicar, a new form of antihyperglycemic agent, through the selective and reversible inhibition of the liver isoform of carnitine palmitoyl-transferase 1. Investigation of glucose production took place in isolated hepatocytes and during pancreatic clamps in healthy rats. The researchers performed chronic treatments on C57BL/6J, db/db, high-fat fed mice, and rats to understand glucose metabolism and insulin sensitivity.
In isolated hepatocytes, teglicar concentration dependently reduced ketone bodies and glucose production up to 72% and 50%, respectively. Antidiabetic activity in hepatocytes and rats was associated with improved insulin sensitivity assessed by the insulin tolerance test. In high-fat fed C57BL/6J mice, long-term teglicar administration normalized glycemia (−19%) and insulinemia (−53%).
Members of the fibroblast growth factor family stimulate glucose uptake and update mitochondrial function in key metabolic tissues [Cantó C, Auwerx J. Science 2012]. FGF21 has become particularly interesting as exongenous administration to animal models of diabetes and obesity is generally associated with weight loss [Muise ES et al. Mol Pharmacol 2008; Kharitonenkov A et al. Endocrinology 2007]. The understanding of FGF21 biology is still evolving.
- © 2012 MD Conference Express®