Summary
A few decades ago, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitide—granulomatosis with polyangiitis, polyangiitis, and microscopic polyangiitis—were associated with high rates of mortality. Tremendous strides have since been made in treating these diseases. The use of less toxic immunosuppressant drugs has improved the outcomes of patients, and, in recent years, the development of biologic agents and a better understanding of disease pathogenesis have contributed to the discovery of rituximab as an effective alternative to the immunosuppressive cyclophosphamide to induce remission in ANCA-associated vasculitis.
- Vasculitis
A few decades ago, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV)—granulomatosis with polyangiitis (GPA), polyangiitis, and microscopic polyangiitis (MPA)—were associated with high rates of mortality. Tremendous strides have since been made in treating these diseases. The use of less toxic immunosuppressant drugs has improved the outcomes of patients, and, in recent years, the development of biologic agents and a better understanding of disease pathogenesis have contributed to the discovery of rituximab as an effective alternative to the immunosuppressive cyclophosphamide to induce remission in ANCA-associated vasculitis. In light of this development, the roles of cyclophosphamide and rituximab in the treatment of AAV, both in remission induction and maintenance of remission, are evolving.
The History of B Lymphocytes as a Target
Ulrich Specks, MD, Mayo Clinic, Rochester, Minnesota, USA, discussed targeting B lymphocytes in the treatment of AAV. B lymphocytes have been a target in GPA since cyclophosphamide was shown to have a significant effect on suppressing the function of B lymphocytes in patients with the disease [Cupps TR et al. J Immunol 1982]. Later, the activity and extent of GPA was shown to be directly linked to the frequency of activated peripheral blood B lymphocytes [Popa ER et al. J Allergy Clin Immunol 1999]. In 1999, a patient with refractory disease was treated successfully with rituximab [Specks U et al. Arthritis Rheum 2001], and this success was reproduced in a small cohort of patients treated with rituximab on a compassionate-use basis [Keogh KA et al. Arthritis Rheum 2005].
Early success with rituximab in uncontrolled trials encouraged a group of investigators to compare the efficacy of rituximab with cyclophosphamide for induction of remission in AAV. Rituximab was approved for the treatment of patients with GPA or MPA on the basis of this study, the Rituximab in Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis [RAVE; Stone JH et al. N Engl J Med 2010] trial.
The multicenter, randomized, double-blind, double-dummy, controlled, noninferiority trial compared rituximab with cyclophosphamide for the induction of complete remission by 6 months in patients with severe AAV. A total of 197 subjects with active severe GPA or MPA were randomized to receive either intravenous (IV) rituximab (375 mg/m2 1x/week for 4 weeks, plus placebo cyclophosphamide; n=99), or oral cyclophosphamide (placebo rituximab infusions followed by 2 mg/kg cyclophosphamide QD for 3 to 6 months; n=98). The study excluded subjects who were in the ICU, had alveolar hemorrhage, or had creatinine >4 mg/dL.
The primary endpoint was complete clinical remission, defined as a Birmingham Vasculitis Activity Score for Wegener's granulomatosis of 0 and complete tapering of prednisone dose at 6 months.
Subjects in the cyclophosphamide arm who reached remission at 3 to 6 months were eligible to be switched to azathioprine (2 mg/kg QD). Subjects in the rituximab group who achieved remission at 3 to 6 months were switched to placebo-azathioprine. Both treatment groups received 1 to 3 pulses of methylprednisone (1000 mg each) at the start of treatment, followed by prednisone (1 mg/kg QD). Doses were tapered so that subjects in remission without disease flares had discontinued glucocorticoids by 5 months.
Sixty-three subjects in the rituximab group (64%) reached the primary endpoint, compared with 52 (53%) in the control group, which met the criterion for noninferiority (p<0.001). There was no significant difference between treatment arms on the primary endpoint by baseline renal status, ANCA type, or disease type (GPA vs MPA), or in those with alveolar hemorrhage at baseline. RAVE demonstrated that rituximab is noninferior to cyclophosphamide for induction of remission in patients with severe AAV. For the subgroup of patients who had a severe disease relapse at baseline of the trial (n=101), rituximab was shown to be superior to cyclophosphamide (p=0.013). While RAVE showed noninferiority for primary outcome measure, a benefit for rituximab was shown for subjects who relapsed. The drop in B cells did not seem to bear any relation to the treatment effect, and the cyclophosphamide group also showed a reduction in B cells.
A second randomized controlled trial comparing a rituximab-based regimen with a standard cyclophosphamide/azathioprine-based regimen in the treatment of active generalized AAV [RITUXVAS] was an international trial with an open-label design of 44 patients who were randomized 3:1 to rituximab plus 2 infusions of cyclophosphamide or IV cyclophosphamide for 6 months followed by oral azathioprine [Jones RB et al. N Engl J Med 2010]. All subjects had a new diagnosis of ANCA-positive vasculitis at entry and had severe renal disease. On average, they were 10 years older than patients enrolled in RAVE, and 25% had received plasma exchange immediately prior to randomization.
The primary endpoint, sustained remission at 12 months, was achieved by 76% of the rituximab group versus 82% of the cyclophosphamide group in an intention-to-treat analysis. RITUXVAS demonstrated that over 12 months, 1 course of rituximab achieved the same results as 6 months of cyclophosphamide followed by azathioprine.
Remission maintenance was again shown to be similar between the 2 strategies in an analysis of 18-month follow-up of RAVE participants. In RAVE, the duration of complete remission was not different between 1 course of rituximab and 18 months of standard therapy (3 to 6 months of cyclophosphamide followed by azathioprine).
A Continued Role for Cyclophosphamide
Cyclophosphamide continues to have a role in remission induction in patients with GPA and MPA, as well as for many severe forms of vasculitis, according to Carol A. Langford, MD, MHS, Cleveland Clinic, Cleveland, Ohio, USA.
Dr. Langford stated that 40 years of learning experience with cyclophosphamide have led to refinement in the way it is used and an improvement in outcomes, as well as specific strategies to reduce toxic side effects.
After the initial 15 patients with GPA were treated successfully with cyclophosphamide in 1973 [Fauci AS, Wolff SM. Medicine 1973], with 12 achieving remission for up to 63 months, cyclophosphamide in combination with corticosteroids was used in more patients who were followed over longer periods of time. Experience with the use of cyclophosphamide over the years has shown that GPA has the potential for relapse and is associated with significant toxicity, including a high rate of bladder cancer and a potential risk of serious infections. The risk of bladder cancer with cyclophosphamide is related to total dose and duration of exposure, with a 5% risk at 10 years from the first cyclophosphamide dose to 15% at 16 years [Talar-Williams C et al. Ann Intern Med 1996]. In a 1995 study of 180 GPA patients, a 6% rate of Pneumocystis jiroveci (PCP) was observed with cyclophosphamide treatment. One lesson from this study is that PCP prophylaxis should be given to patients with GPA/MPA receiving an induction regimen, including rituximab.
Cyclophosphamide use has evolved, and treatment is now considered in 2 phases: induction of remission followed by maintenance. Azathioprine maintenance after 3 to 6 months of cyclophosphamide induction was found to maintain remission without an increase in the relapse rate [Jayne D et al. N Engl J Med 2003], and methotrexate maintenance following cyclophosphamide induction was found similarly effective as azathioprine in maintenance [Pagnoux C et al. N Engl J Med 2008].
The time to remission induction is equivalent between intermittent IV and daily oral cyclophosphamide at a median of 3 months, but a higher rate of relapse has been observed with IV intermittent therapy [de Groot K et al. Ann Intern Med 2009]. However, patients who receive daily therapy receive a higher total dose and have a higher rate of leukopenia, leading to the recommendation that complete blood count should be monitored every 1 to 2 weeks for the duration of daily therapy.
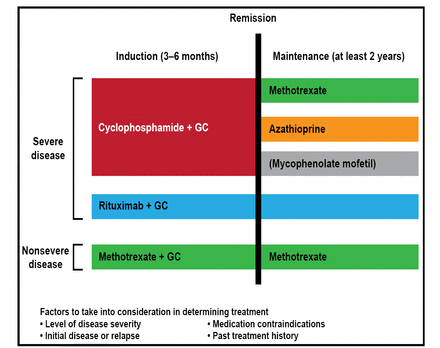
Another lesson learned is that nonsevere disease can be successfully treated with alternatives to cyclophosphamide, such as methotrexate (Figure 1) [Hoffman GS et al. Arthritis Rheum 1992; Sneller MC et al. Arthritis Rheum 1995]. In a study of 100 patients with nonsevere disease, methotrexate was not inferior to cyclophosphamide for remission induction [de Groot K et al. Arthritis Rheum 2005].
2012 Treatment of GPA and MPA.
GC=glucocorticoid; GPA=granulomatosis with polyangiitis; MPA=microscopic polyangiitis.
Reproduced with permission from CA Langford, MD, MHS.
Evidence from the literature supports improved outcomes with cyclophosphamide over time. In a German cohort of 445 patients observed over 4 decades, mortality declined over time, owing in part to improved use of cyclophosphamide [Holle JU et al. Arthritis Rheum 2011]. In addition to Pneumocystis prophylaxis and cytopenia prevention mentioned previously, the changes in cyclophosphamide use include the following:
-
Limiting the duration of exposure to 3 to 4 months
-
Urothelial protection in the form of once morning dosing and adequate fluids to maintain a dilute urine for daily cyclophosphamide, and the use of mesna in conjunction with intermittent cyclophosphamide
-
Urinalysis to detect nonglomerular hematuria and urine cytology
-
Cystoscopy for nonglomerular hematuria or atypia seen on urine cytology
Dr. Langford said that, in the end, the data support cyclophosphamide as an equally valid option to rituximab in newly diagnosed patients with severe disease. The current body experience with cyclophosphamide favors its use over rituximab in patients with rapidly progressive glomerulonephritis with creatinine >4.0 mg/dL, patients with alveolar hemorrhage requiring mechanical ventilation—as these patients were not included in the RAVE trial—when they experience adverse events specific to rituximab, and patients with active disease despite rituximab.
The editors would like to thank the many members of the American College of Rheumatology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2012 MD Conference Express®