Summary
Proprotein convertase subtilisin kexin type 9 (PCSK9) is a circulating protein that plays a pivotal role in cholesterol homeostasis by reducing expression of low-density lipoprotein cholesterol (LDL-C) receptors by the liver, leading to diminished hepatic clearance capacity for plasma LDL-C and increased LDL-C levels in the blood [Cohen JC et al. N Engl J Med 2006; Lagace TA et al. J Clin Invest 2006; Benjannet S et al. J Biol Chem 2010]. AMG 145 is a fully human monoclonal antibody that blocks PCSK9 binding to the LDL receptor, improving LDL-C clearance and reducing circulating LDL-C concentration [Raal F et al. Circulation 2012].
- Cardiology Clinical Trials
- Featured Meeting - Specialty page
- Lipid Disorders
Proprotein convertase subtilisin kexin type 9 (PCSK9) is a circulating protein that plays a pivotal role in cholesterol homeostasis by reducing expression of low-density lipoprotein cholesterol (LDL-C) receptors by the liver, leading to diminished hepatic clearance capacity for plasma LDL-C and increased LDL-C levels in the blood [Cohen JC et al. N Engl J Med 2006; Lagace TA et al. J Clin Invest 2006; Benjannet S et al. J Biol Chem 2010]. AMG 145 is a fully human monoclonal antibody that blocks PCSK9 binding to the LDL receptor, improving LDL-C clearance and reducing circulating LDL-C concentration [Raal F et al. Circulation 2012]. In Phase 1 studies, AMG 145 was well tolerated and significantly reduced LDL-C in healthy subjects and in subjects with hypercholesterolemia, including those with heterozygous familial hypercholesterolemia (HeFH) [Dias C et al. J Am Coll Cardiol 2012], a common inherited disease characterized by markedly elevated LDL-C, which if left untreated is associated with significant premature cardiovascular (CV) mortality and morbidity.
Results from the Study to Assess the Tolerability and Efficacy of AMG 145 in Patients with Heterozygous Familial Hypercholersterolemia [RUTHERFORD] presented by Frederick Raal, MD, University of Witwatersrand, Johannesburg, South Africa, showed that AMG 145 produced rapid and sustained reductions in LDL-C in HeFH patients receiving statins, with or without ezetimibe.
The RUTHERFORD study was a global, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of subcutaneous (SC) AMG 145 (350 mg and 420 mg) administered every 4 weeks (Q4W) in a large and diverse cohort of HeFH patients unable to achieve an LDL-C <100 mg/dL despite statin therapy, with or without ezetimibe. The trial included patients (n=168; mean age ∼50 years) diagnosed with HeFH with an LDL-C ≥100 mg/dL and triglycerides ≤400 mg/dL, who were being treated with stable lipid-lowering therapy (statin, ezetimibe, bile acid sequestrants, or niacin). Subjects were randomly assigned to AMG 145 at 350 or 420 mg SC Q4W, or placebo for 12 weeks. The primary study endpoint was percent change in LDL-C measured by ultracentrifugation, from baseline at 12 weeks.
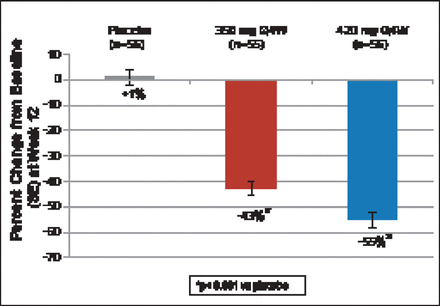
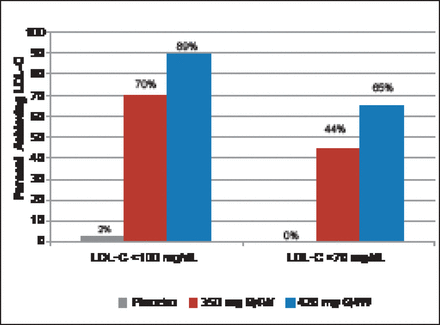
At 12 weeks, patients treated with 350 and 420 mg of AMG 145 had a 43% (p<0.001) and 55% (p<0.001) decrease in LDL-C, respectively, compared with a 1% increase with placebo (Figure 1). Significant decreases were seen beginning at Week 2. At Week 12, 70% and 89% of patients achieved LDL-C levels <100 mg/dL with AMG 145 at 350- and 420 mg, respectively. Levels of <70 mg/dL were achieved in 44% and 65% of subjects, respectively (Figure 2). Significant reductions in apolipoprotein (Apo)B, total cholesterol (TC), very low LDL-C (VLDL-C), non-high-density lipoprotein-cholesterol (non-HDL-C), triglycerides, and lipoprotein (Lp[a]), and an increase in HDL-C but not ApoA1 were also seen.
Change in LDL-C from Baseline to Week 12.
ANCOVA=analysis of covariance; Q4W=every 4 weeks; SE=standard error; UC=ultracentrifugation. LDL-C values at baseline and Week 12 were measured using preparative UC. Least Square Means are presented from the ANCOVA model including treatment and stratification factors as covariates. Missing UC LDL-C values at Week 12 were imputed using last observation carried forward and calculated LDL-C. A Hochberg adjustment was used to control the family wise error rate at ≤0.05.
Reproduced with permission from F Raal, MD.
Percentage of Patients Achieving LDL-C Targets by Week 12.
LDL-C=low-density lipoprotein; Q4W=every 4 weeks.
Reproduced with permission from F Raal, MD.
AMG 145 was well tolerated. There was no difference in the number of adverse events (AEs) between AMG 145 and placebo. The most common AEs were nasopharyngitis, injection-site pain, and headache. Two serious AEs were noted in the 420-mg treatment group but were considered nontreatment related. Three patients experienced creatine kinase elevations with active treatment. These were asymptomatic and resolved spontaneously. These results suggest that AMG 145 may offer a new effective treatment option to further reduce LDL-C in HeFH patients unable to achieve optimal LDL-C targets on current medications [Raal F et al. Circulation 2012].
Evan A. Stein, MD, PhD, Metabolic and Atherosclerosis Research Center, Cincinnati, Ohio, USA, reported that SC administration of AMG 145 significantly reduced LDL-C levels comparable to those achieved with the most efficacious statins in statin-intolerant patients.
The objective of the Study to Assess the Tolerability and Efficacy of AMG 145 in Patients with Hypercholesterolemia Unable to Tolerate an Effective Dose of a Statin [GAUSS] was to assess the efficacy and tolerability of AMG 145 versus ezetimibe in adult patients aged 18 to 75 years at CV risk and with statin intolerance due to muscle-related (intolerable myalgia or myopathy) side effects. It was a 12-week, global, double-blind, randomized, placebo-controlled study. Patients (n=160) were randomly assigned equally to 1 of 5 groups: AMG 145 Q4W alone at doses of 280, 350, or 420 mg; AMG 145 at 420 mg plus ezetimibe 10 mg QD; or ezetimibe 10 mg QD plus placebo Q4W. AMG 145 or placebo was administered at Day 1, and Weeks 4 and 8. The primary endpoint was the change in LDL-C, measured by ultracentrifugation, from baseline at Week 12. Other endpoints included safety and tolerability of different doses of AMG 145 alone and with ezetimibe. Subjects were mean age 62 years; 64% were women. All patients had intolerance to 1 or more statins.
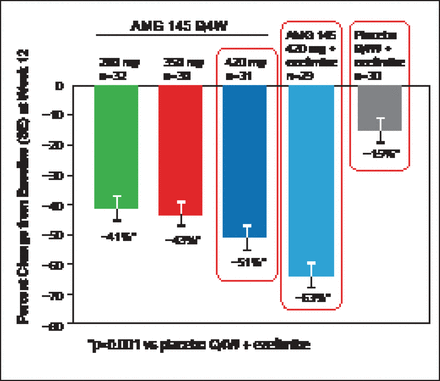
At Week 12, mean changes in LDL-C levels were dose-dependent and significant: −67 mg/dL (−41%; 95% CI, −49% to −33%) with AMG 145 at 280 mg; −70 mg/dL (−43%; 95% CI, −51% to −35%) with 350 mg; −91 mg/dL (−51%; 95% CI, −59% to −43%) with 420-mg; and −110 mg/dL (−63%; 95% CI, −71% to −55%) for the 420 mg plus ezetimibe group compared with −14 mg/dL (−15%; 95% CI, −23% to −7.0%) for the placebo plus ezetimibe group (all p<0.001 vs placebo plus ezetimibe; Figure 3) [Sullivan D et al. JAMA 2012]. Calculated LDL-C was reduced by Week 2 for all doses of AMG 145. At Week 12, 90% and 62% of patients treated with AMG 145 at 420 mg plus ezetimibe achieved the LDL-C goal of <100 mg/dL and <70 mg/dL, respectively. Reductions in other lipid parameters including Lp(a) were also seen with AMG 145.
Percent Change in LDL-C from Baseline at Week 12.
Q4W=every 4 weeks; SE=stantard error.
Reproduced with permission from E Stein, MD, PhD.
The most common AEs were myalgia, nasopharyngitis, nausea, and fatigue. There were few serious AEs but none were considered treatment related. Creatine kinase elevations unrelated to treatment occurred in 2 patients [Sullivan D et al. JAMA 2012].
After 12 weeks of treatment with AMG 145, patients with hypercholesterolemia on a stable regimen of a statin, with or without ezetimibe, experienced an improved lipid profile, reported Robert Giugliano, MD, SM, Brigham and Women's Hospital, Boston, Massachusetts, USA.
The LDL-C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy [LAPLACE-TIMI 57] trial—the largest with PCSK9 mAb conducted to date—was a 12-week, randomized, double—blind, dose-ranging, placebo-controlled Phase 2 study to compare the efficacy of AMG 145 given SC either every 2 weeks (Q2W) or Q4W with placebo in stable patients with hypercholesterolemia currently taking a statin, with or without ezetimibe. The primary study endpoint was percentage change in LDL-C measured using ultracentrifugation. Secondary outcomes included changes in other lipoproteins, pharmacokinetics, pharmacodynamics, and tolerability and safety.
Patients aged 18 to 80 years on stable doses of a statin (with or without ezetimibe) for 4 weeks with fasting LDL-C levels ≥85 mg/dL and fasting triglycerides ≤400 mg/dL were eligible. Subjects were also required to be free of other major comorbidities; to not be on other prescription lipid lowering therapy; and to not have had recent acute coronary syndrome, revascularization, or stroke.
Patients (n=934) were screened, which included fasting LDL-C safety laboratory assessment and an SC placebo injection of 6 mL of normal saline (administered as 3 injections of 2 mL) to assess tolerability. Eligible subjects (n=631) were then randomly assigned to 1 of the following groups: Q2W groups (placebo, AMG 145 at 70, 105, or 140 mg) or Q4W groups (placebo, or AMG 145 at 280-, 350-, or 420 mg). Patients in the Q2W groups received AMG 145 or placebo on Day 1, and Weeks 2, 4, 6, 8, and 10; patients in the Q4W groups were treated on Day 1, and Weeks 4 and 8. Randomization was stratified by baseline LDL levels (<130 vs ≥130 mg/dL) and the use of ezetimibe. Details of this study's design have been published previously [Kohli P et al. Clin Cardiol 2012].
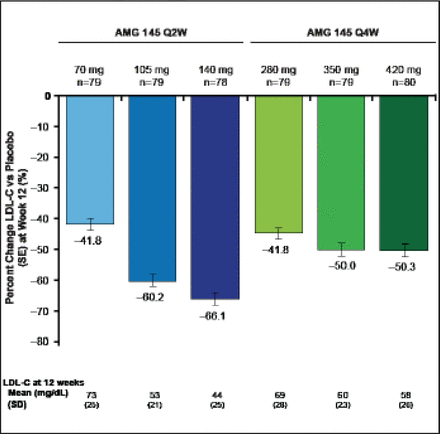
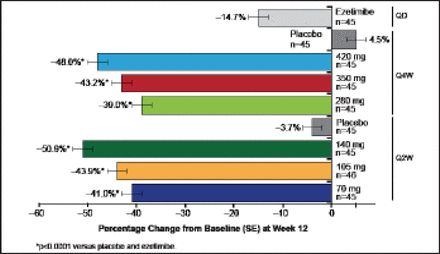
Treated subjects (n=631) were a mean of age 61 years, and 51% were women. Mean LDL was 123 mg/dL, 9% of subjects were on ezetimibe, and 29% were being treated with an intensive statin regimen. All doses of AMG 145 significantly reduced LDL-C (p<0.0001 vs placebo; Figure 4). The reductions in LDL-C at the end of the dosing intervals ranged from 42% to 66% for the Q2W regimens and from 42% to 50% for the Q4W regimens. Mean acheved LDL-C measured by ultracentrifugation at Week 12 ranged from 73 to 44 mg/dL for the Q2W doses and 69 to 58 mg/dL for the Q4W doses. Findings were consistent across major subgroups.
Primary Endpoint: AMG 145 Reduced LDL-C at 12 Weeks.
LDL-C=low-density lipoprotein cholesterol; Q2W=every 2 weeks. SE=standard error. p<0.0001 for each dose vs placebo.
Reproduced with permission from RP Giugliano, MD.
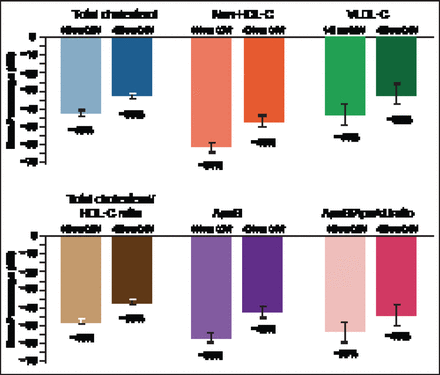
Response was rapid—occurring at 2 weeks with both dosing regimens–and lasted to Week 12 (p<0.0001 for all measurement periods). There was some upward movement over the dosing interval with the Q4W dosing due to the timing of measurement that occurred at 2 week intervals. Significant reductions (p<0.0001 vs placebo) were seen for TC, non-HDL-C, VLDL-C, TC/HDL-C, ApoB, and ApoB/ApoA1, with both top AMG 145 doses (140 mg Q2W and 420 mg Q4W; Figure 5). There were no dose-related or treatment-related AEs or serious AEs. Importantly there was no antibody formation in this study in patients receiving AMG 145.
Secondary Results at 12 Weeks with Top Two AMG 145 Doses.
LDL-C=low-density lipoprotein cholesterol; HDL-C=high-density lipoprotein cholesterol; VLDL-C=very-low-density lipoprotein cholesterol; Q4W=every 4 weeks. p<0.0001 versus placebo for all parameters.
Reproduced with permission from RP Giugliano, MD.
The results suggest that PCSK9 inhibition could be a new model in lipid management [Giugliano R et al. Lancet 2012]. Inhibition of PCSK9 and impact on clinical endpoints warrants assessment in Phase 3 clinical trials.
Michael J. Koren, MD, Jacksonville Center for Clinical Research, Jacksonville, Florida, USA, presented the results of the Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid Levels [MENDEL] trial, a Phase 2 study of the efficacy and safety of AMG 145 as monotherapy for hypercholesterolemia. The results showed that AMG 145 significantly reduced serum LDL-C versus placebo and ezetimibe [Koren MJ et al. Lancet 2012].
Adults not currently on lipid-lowering therapy with LCL-C ≥100 but <190 mg/dL and 10-year Framingham coronary heart disease risk score ≤10% were randomly assigned to 1 of 9 treatment arms: Q2W groups (placebo, or AMG 145 at 70, 105, or 140 mg), Q4W groups (placebo, or AMG 145 at 280, 350, or 420 mg), or oral ezetimibe QD. The primary study endpoint was percentage change in LDL-C by ultracentrifugation from baseline at 12 weeks. Subjects (AMG 145, n=271; placebo, n=90; ezetimibe, n=45) were mean a age of 50 years and mostly white women. Mean baseline LDL-C was between 140 and 145 mg/dL; mean baseline PCSK9 levels were between 341 and 350 ng/mL.
At Week 12, there was a significant dose-dependent reduction in LDL-C levels with both dosing regimens at all doses (Figure 6). Reductions were rapid, with the highest AMG 145 doses leading to the greatest reductions (51% with 140 mg Q2W and 48% with 420 mg Q4W compared with virtually no change with placebo and 15% with ezetimibe). Results were maintained throughout the study and did not vary among any of the prespecified subgroups. As in other studies, treatment with AMG 145 also led to significant reductions in TC, non-HDL-C, ApoB, TC/HDL-C ratio, ApoB/ApoA1 ratio and Lp(a). AEs were similar to those seen in previous trials, and AMG 145 was well tolerated.
Effects of AMG 145 Versus Placebo and Ezetimibe on LDL-C.
LDL-C=low-density lipoprotein cholesterol; Q2W=every 2 weeks; Q4W=every 4 weeks; QD=every day; SE=standard error.
Adapted from MJ Koren, MD. AHA 2012.
Integrating the trials of PCSK9 inhibition for lipid lowering, all have shown that these agents are well tolerated and have potent lipid-lowering effects. This mechanism of therapy has the potential to get substantially more patients to LDL goals when added to currently available therapies [Giugliano RP et al. Lancet 2012; Kohli P et al. Clin Cardiol 2012]. Well-powered clinical outcome trials are needed in order to evaluate whether LDL lowering through PCSK9 inhibition leads to reductions in adverse CV events.
- © 2012 MD Conference Express®