Summary
Since its approval in 1997, clopidogrel has been a cornerstone of therapy in patients with acute coronary syndrome and in those patients who are undergoing percutaneous coronary intervention. Although the drug reduces the risk of future ischemic events, high variability in the antiplatelet effect between patients has been demonstrated. A number of factors are responsible for this variability, including clinical factors, drug interactions, and the presence of CYP2C19 loss-of-function alleles that hinder the metabolism of clopidogrel to its active form, thus leading to attenuation of its antiplatelet effect [Brandt JT et al. J Thromb Haemost 2007].
- Thrombotic Disorders
- Coronary Artery Disease Genomics
Since its approval in 1997, clopidogrel has been a cornerstone of therapy in patients with acute coronary syndrome (ACS) and in those patients who are undergoing percutaneous coronary intervention (PCI). Although the drug reduces the risk of future ischemic events, high variability in the antiplatelet effect between patients has been demonstrated. A number of factors are responsible for this variability, including clinical factors, drug interactions, and the presence of CYP2C19 loss-of-function alleles that hinder the metabolism of clopidogrel to its active form, thus leading to attenuation of its antiplatelet effect [Brandt JT et al. J Thromb Haemost 2007].
The antiplatelet effect of clopidogrel is most commonly assessed by measuring adenosine dipohosphate (ADP)-induced platelet aggregation (the phenotype). Testing for the CYP2C19 genotype also predicts the antiplatelet response. Deepak L. Bhatt, MD, MPH, Brigham and Women's Hospital, Boston, Massachusetts, USA, said that the advantage of genetic testing is that the results are fixed; however, the genome is distanced from the phenotype. In contrast, platelet function testing is more proximal to the phenotype and captures environmental and genetic variability, but it is more difficult to assess than genetic testing and varies over time.
The discovery of the association between the CYP2C19 genotype and poor response to clopidogrel led researchers to explore whether genetic testing or platelet function testing could help tailor antiplatelet therapy and also prompted the US Food and Drug Administration (FDA) to issue a boxed warning on the clopidogrel package insert in March 2010.
The FDA Boxed Warning
Michael Pacanowski, PharmD, MPH, Center for Drug Evaluation and Research, FDA, Silver Spring, Maryland, USA, explained that the Code of Federal Regulations requires that a warning must be presented in a box when the hazard presented by the drug may lead to death or serious injury. The warning was designed to alert prescribing physicians that higher rates of cardiovascular (CV) events had been found among people who were considered to be poor metabolizers of clopidogrel. Dr. Pacanowski said that the warning targets carriers of 2 CYP2C19 loss-of-function alleles, because at the time, the most robust evidence was available for that population; however, carriers of only 1 CYP2C19 loss-of-function allele have also shown to be at higher risk than patients who carry 2 wild-type alleles. The warning is restricted to the settings of ACS/PCI, as data are lacking for the setting of stroke and peripheral artery disease.
The warning also notes that tests are available to identify genetic differences in CYP2C19 function and advises that health care professionals consider the use of other antiplatelet medications or alternative dosing strategies for clopidogrel in patients who are identified as poor metabolizers.
Guidance from ACCF/AHA
Three months after the FDA's announcement about the boxed warning, the ACCF/AHA issued a joint Clinical Alert [Holmes DR Jr et al. JACC 2010]. The goal of the alert was to provide guidance, education, and a framework for research, said David R. Holmes, Jr., MD, Mayo Clinic, Rochester, Minnesota, USA, the lead author of the alert.

2010 ACCF/AHA Clinical Alert
Recommendations
-
Adherence to existing evidence-based guidelines from the ACC, AHA, or other professional societies for using antiplatelet therapies should remain the foundation of care. If clopidogrel is prescribed, health care providers should help ensure that patients take it as prescribed.
-
Clinicians must be aware that in certain patients with either acute or chronic coronary artery disease, genetic variability in response to clopidogrel can affect its inhibition of platelet function.
-
Careful clinical judgment, including weighing the risks and benefits, is needed in considering all therapies. The new boxed warning points out that for clopidogrel, if there is a lack of efficacy, the consequences can potentially result in fatal outcomes.
-
Results from ongoing clinical trials in large groups of patients will provide more information about the predictive value of genetic testing and better inform the role that genotyping might play in personalizing medicine and optimizing outcomes.
Recommendations for Practice
-
The evidence base is insufficient to recommend either routine genetic or platelet function testing at the present time.
-
There are several possible therapeutic options for patients who experience an adverse event while taking clopidogrel in the absence of any concern about medication compliance.
At the time of the Clinical Alert, the evidence base was insufficient to recommend either routine genetic or platelet function testing, said Dr. Holmes, adding that more robust data are now available. However, some data are conflicting, leaving clinicians uncertain about the benefit of tailoring antiplatelet therapy according to the results of genetic or platelet function testing.
The Debate
The first genomewide association study was seminal work in the area of response to clopidogrel, said Dr. Bhatt. In that study, the CYP2C19 variant not only diminished platelet aggregation but also led to poorer clinical outcomes: a CV ischemic event or death was twice as likely among patients with the genotype (compared with noncarriers) during 1 year of follow-up after PCI [Shuldiner AR et al. JAMA 2009].
Matthew J. Price, MD, Cardiac Catheterization Laboratory, Scripps Clinic, La Jolla, California, USA, described several studies in which poor response to clopidogrel, as measured by ADP-induced platelet aggregation, was associated with a risk of CV events. In one study, poor response was associated with greater than a 2-fold higher rate of death, myocardial infarction (MI), or stroke compared with good response in a population of patients who were undergoing elective PCI [Campo G et al. JACC 2010].
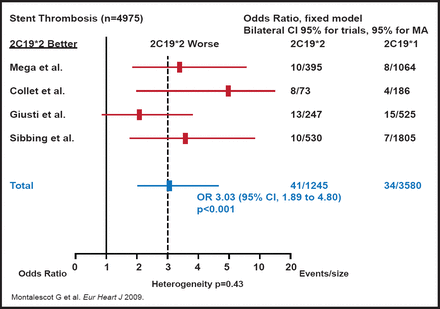
In addition, a meta-analysis showed that the rates of cardiac events and stent thrombosis were significantly higher among carriers of the CYP2C19*2 (or CYP2C19 loss-of-function allele) genotype than noncarriers [Mega JL et al. NEJM 2009]. The greatest effect of poor response to clopidogrel has appeared to be related to stent thrombosis. Stephen D. Wiviott, MD, Brigham and Women's Hospital, Boston, Massachusetts, USA, noted that across studies, there has been a 3-fold increase in stent thrombosis among patients with the CYP2C19*2 genotype who were treated with clopidogrel (Figure 1) [Montalescot G et al. Eur Heart J 2009].
Risk of Stent Thrombosis with Clopidogrel.
Reproduced with permission from Oxford University Press.
While Dr. Wiviott acknowledged that major outcomes trials are lacking, he emphasized the established association between the CYP2C19*2 (or loss-of-function allele) genotype and lower metabolism of clopidogrel, less platelet inhibition, and worse clinical outcomes, especially in the setting of ACS and PCI, and stated, “There is strong associative evidence that suggests value to [the use of tailored antiplatelet therapy].”
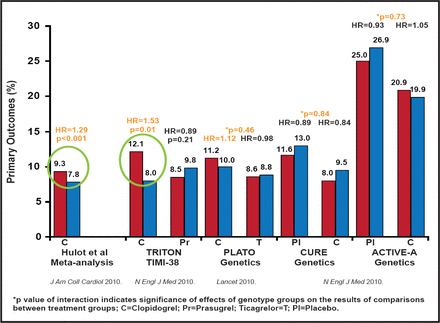
On the other side of the debate, Paul A. Gurbel, MD, Sinai Center for Thrombosis Research, Baltimore, Maryland, USA, pointed to studies that have shown that clopidogrel had clinical benefit that was irrespective of genotype, most notably the study on genotype testing of patients in the CURE and ACTIVE A trials [Pare G et al. NEJM 2010]. He added that the genotype has been linked to risk only in the PCI population (Figure 2).
CYP2C19 Loss-of-Function Allele Carrier Status.
Reproduced with permission from P. Gurbel, MD.
One trial that was designed to evaluate tailored antiplatelet therapy, the GRAVITAS trial, compared the effect of high-dose (150 mg) and standard-dose (75 mg) clopidogrel in patients with high on-treatment platelet reactivity after elective PCI. Compared with standard-dose therapy, high-dose clopidogrel achieved a modest pharmacodynamic effect in patients with high on-treatment platelet reactivity. However, the use of high-dose clopidogrel did not reduce the incidence of death from CV causes, nonfatal MI, or stent thrombosis [Price MJ et al. JAMA2011].
Summarizing his position that the time is not right for tailored antiplatelet therapy, Dr. Gurbel noted, “Thus far, no prospective study has demonstrated that personalizing antiplatelet therapy based on genotype improves clinical outcomes.” He added that studies have shown that antiplatelet effects of newer antiplatelet drugs, such as prasugrel and ticagrelor, are not influenced by the CYP2C19*2 genotype (or loss-of-function allele), and that these drugs may provide alternative treatment strategies.
One year after the ACCF/AHA issued their Clinical Alert, the organizations published an update to their UA/NSTEMI Guidelines, with two new class IIb recommendations regarding the potential lack of response to clopidogrel:
-
Platelet function testing to determine platelet inhibitory response in patients with UA/NSTEMI (or after ACS and PCI) on thienopyridine therapy may be considered if the results of testing may alter management.
-
Genotyping for a CYP2C19 loss-of-function variant in patients with UA/NSTEMI (or after ACS and with PCI) on clopidogrel therapy might be considered if the results of testing may alter management [Wright RS et al. JACC 2011].
The benefit of altering of management, however, remains uncertain, as the cardiology community continues to debate the potential benefit of tailoring antiplatelet therapy.
The editors would like to thank the many members of the American College of Cardiology 60th Annual Scientific Session and i2 Summit presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2011 MD Conference Express