Summary
Ranibizumab, an anti-VEGF antibody fragment that is designed for intraocular use in diabetic retinopathy (DR), led to rapid and sustained improvement in both vision and retinal anatomy, reduced progression of DR, and improved patient-reported function in the RISE and RIDE clinical trials.
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
- Retinal Diseases
Diabetic retinopathy (DR) is the most common cause of blindness in the working populations of the United States and Europe [Sanchez CI et al. Invest Ophthalmol Vis Sci 2011] and is often accompanied by diabetic macular edema (DME). Ranibizumab (RBZ), an anti-VEGF antibody fragment that is designed for intraocular use in DR, led to rapid and sustained improvement in both vision and retinal anatomy, reduced progression of DR, and improved patient-reported function in two major clinical trials in Latin America and Argentina. David Boyer, MD, Retina-Vitreous Associates Medical Group, Los Angeles, California, USA, presented findings from these trials.
RISE (NCT00473330) and RIDE (NCT00473382) were two methodically identical multicenter, randomized, controlled trials for the treatment of DR. The primary endpoint for each double-prospective, double-blind, Phase 3 trial was the proportion of subjects who gained ≥15 standardized eye chart letters (3 lines) in best corrected visual acuity (BCVA) at Month 24. Additional efficacy endpoints included changes in other measures of visual acuity and visual function, changes in retinal anatomy, use of a macular focal/grid laser, and patient-reported visual function/quality of life.
Key inclusion criteria included age ≥18 years with a decrease in vision due primarily to DME; center subfield thickness (CST) ≥275 μm; and study eye BCVA of 20/40 to 20/320. Key exclusion criteria included use of antiangiogenic drugs in either eye within the 3-month period prior to the start of the study; panretinal photocoagulation, macular laser, intraocular steroids, or surgery in the study eye; cerebral vascular accident or myocardial infarction; active proliferative diabetic retinopathy or uncontrolled glaucoma in the study eye; and HbA1C >12.
Patients with loss of vision (BCVA 20/40 to 20/320 Snellen equivalent) and DME on retinal imaging were randomized 1:1:1 to receive monthly RBZ (0.5 mg or 0.3 mg) or placebo injections. Subjects were eligible for rescue macular laser, starting at Month 3. Of the placebo patients, 72% received laser versus 33% of RBZ patients. Ocular and systemic adverse events (AEs) were assessed.
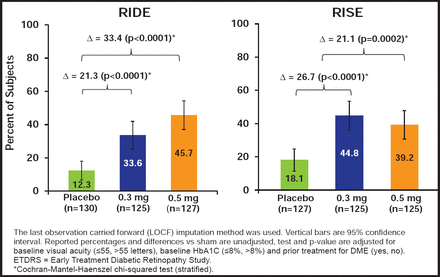
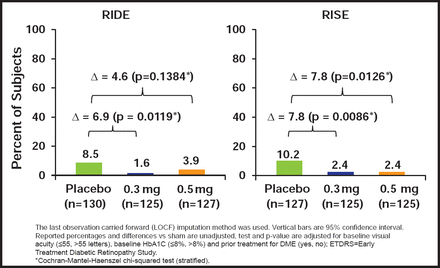
RBZ-treated patients had rapid, sustained, and statistically significant improvements in BCVA and retinal thickness. In both studies, more RBZ-treated patients gained ≥15 letters (Figure 1). Fewer patients had significant visual acuity (VA) loss of ≥15 letters (Figure 2). More patients had a BCVA Snellen equivalent of 20/40 or better at Month 24. Also, fewer patients developed additional complications of DR, and AEs were consistent with prior studies of RBZ.
Primary Endpoint: Proportion of Subjects Gaining ≥15 ETDRS Letters from Baseline to Month 24.
Reproduced with permission from S. Boyer, MD.
Proportion of Subjects Losing ≥15 ETDRS Letters from Baseline to Month 24.
Reproduced with permission from S. Boyer, MD.
DR is the most common microvascular complication of diabetes [Fong DS et al. Diabetes Care 2004] and increases with duration of the disease. Approximately 25% of all type 2 diabetes patients will develop DR [Ciulla TA et al. Diabetes Care 2003]. DME, swelling of the central retina that causes vision loss, occurs in approximately 10% of patients with diabetes. Advanced DR, especially DME, is among the leading causes of blindness in the elderly [Antonetti DA et al. Diabetes 2006].
Laser photocoagulation is standard treatment for DME, but vision improvement after treatment is uncommon. Intraocular use of RBZ may represent an important therapeutic advance by improving vision and reducing the severity of retinopathy in DME.
- © 2011 MD Conference Express