Summary
Although the side effects that are associated with statin use are limited, the risk of adverse events becomes more significant as the dose increases when they are used in combination with other medications and in certain patient populations (pregnant women, the elderly, and children). This article discusses several of the at-risk populations and how to treat them.
- lipid disorders
Although the side effects that are associated with statin use are limited, the risk of adverse events (AEs) becomes more significant as the dose increases when they are used in combination with other medications and in certain patient populations (pregnant women, the elderly, and children). Ashraf Reda, MD, Menofiya University, Shibin el Kom, Egypt, discussed several of the at-risk populations and how to treat them.
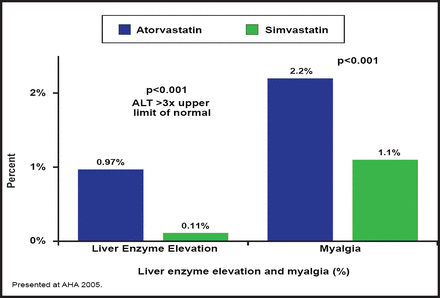
Recent trials have demonstrated the clinical benefits of high-dose statins; however, they have also shown that higher doses are often accompanied by an increased incidence of side effects. The Treat to New Target (TNT) trial was the first large randomized clinical trial to compare high-dose (80 mg) and moderate-dose (10 mg) atorvastatin as secondary prevention in patients with established stable coronary heart disease (CHD). In TNT, treatment with 80 mg atorvastatin resulted in significant benefits—eg, 22% relative risk reduction in cardiovascular (CV) events (p=0.001) and a 25% relative reduction in stroke risk (p=0.02) compared with the 10-mg dose; however, the benefits occurred in combination with a significantly greater incidence of elevated aminotransferase levels (1.2% vs 0.2%; p<0.001) [LaRosa JC et al. N Engl J Med. 2005]. Similar findings were reported in the Incremental Decrease in Clinical Endpoints Through Aggressive Lipid Lowering (IDEAL) trial, which reported more frequent AEs and liver enzyme elevations with 80 mg atorvastatin compared with 20 mg simvastatin (Figure 1). Prof. Reda noted that at least in large trials, elevated liver enzymes occur in less than 1% of the treated population, are usually asymptomatic, rarely cause liver failure, and usually improve with continuing statin use or by reducing the dose. Switching to another statin may also help.
IDEAL Trial: Serious AEs.
Reproduced with permission from A. Reda, MD.
Similarly, in the SEARCH trial [SEARCH Collaborative Group. Lancet 2010], simvastatin 80 mg had higher rates of liver enzyme elevation and muscle-related toxicity (including rhabdomyolysis) compared with 20 mg simvastatin.
The elderly, patients with a low body mass index, those with hepatic or renal dysfunction, and/or patients who take concomitant medications or combination therapy are at a heightened risk for statin-related myalgia (common), myopathy (much less common), and more seriously, but rarely, rhabdomyolysis. The risk of myopathy and rhabdomyolysis is also increased with use of a fibrate and statin in combination. On June 10, 2011, after reviewing the SEARCH trial data and comparing these results with prior studies, the United States Food and Drug Administration restricted the use of simvastatin 80 mg due to an increased risk of rhabdomyolysis and recommended lower doses of simvastatin in combination with drugs that interfere with the metabolism of simvastatin via the cytochrome P450 3A4 system (eg, amiodarone, verapamil, diltiazem, amlodipine, ranolazine).
To minimize these muscle side effects, Prof. Reda recommended using statins alone to achieve non-high-density lipoprotein cholesterol (HDL-C) treatment goals; using fish oils or niacin rather than fibrates; using the lowest effective dose of both the statin and fibrate; cautiously dosing statins that are renally cleared (eg, simvastatin, lovastatin, pravastatin) in patients who have compromised kidney function; avoiding specific statins at high doses that are associated with higher rates of rhabdomyolysis (eg, simvastatin 80 mg); and obtaining a baseline creatine kinase (CK) level and repeating it during the course of therapy if the patient reports muscle symptoms. Treatment should be discontinued in the presence of muscle symptoms and a CK >10 times the upper limit of normal.
Although there have been no large well-controlled studies, taking CoQ10 supplements may correct the negative effects that are caused by statins without affecting their positive effects on cholesterol levels and may reduce statin-induced muscle symptoms. Statin and fibrate use should be stopped for pregnant or lactating patients. While fibrates are tumorigenic for the fetus, statin use is effective and safe for children aged ≥8 years with familial dyslipidemia. Prof. Reda recommends 20 mg pravastatin until the age of 14 years and 40 mg thereafter.
The occurrence of side effects with one statin does not mean that another statin should not be used. Before beginning a treatment protocol, Prof. Reda recommends using a validated questionnaire, including family history to document statin intolerance, and measurement of CK, renal, and thyroid function. Other studies, including genetic testing for statin efficacy and potential toxicity, that evaluate proximal muscle strength and perform a percutaneous muscle biopsy may be considered in selected patients.
Atherosclerosis is a chronic inflammation that is induced by increased levels of cholesterol. Preserved endothelial function attenuates the risk of future coronary events that are associated with a high plaque burden and high levels of cholesterol. Thomas F. Lüscher, MD, University of Zurich, Zurich, Switzerland, described the life cycle of coronary artery disease (CAD) and the role of lipids in the management of CAD.
The benefits of early reduction in low-density lipoprotein cholesterol (LDL-C) were shown in the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE IT) trial, which compared 40 mg of pravastatin daily (standard therapy) with 80 mg of atorvastatin daily (intensive therapy). The primary endpoint of PROVE IT was a composite of death from any cause, myocardial infarction (MI), documented unstable angina that required rehospitalization, revascularization (performed at least 30 days after randomization), and stroke. Kaplan-Meier estimates of the rates of the primary endpoint at 2 years were 26.3% in the pravastatin group and 22.4% in the atorvastatin group, reflecting a 16% relative risk reduction; hazard ratio in favor of atorvastatin (p=0.005; 95% CI, 5 to 26) [Cannon CP et al. New Engl J Med 2004].
The Atorvastatin for Reduction of MYocardial Damage During Angioplasty—Acute Coronary Syndromes (ARMYDA-ACS) trial suggested that statins have an anti-inflammatory effect and that that even short-term pretreatment with atorvastatin may improve outcomes in patients with ACS who are undergoing early invasive strategy. Elevations in creatine kinase-myocardial band (CK-MB), troponin-I, and C-reactive protein (CRP) were all significantly (p≤0.01) reduced in patients who were pretreated with atorvastatin 80 mg [Patti G et al. J Am Coll Cardiol 2007].
Despite worries that high doses of statins may be associated with more serious adverse events, safety studies have shown that they are quite safe at doses up to 80 mg. However, Prof. Thomas F. Lüscher recommends checking lipids, liver enzymes, and CK-MB after 8 weeks. If liver enzymes and/or CK are elevated, change the product.
Results of the Collaborative Atorvastatin Diabetes Study (CARDS) showed that in patients with diabetes but no atherosclerosis, atorvastatin 10 mg daily is safe and efficacious in reducing the risk of first cardiovascular disease event, including stroke [Colhoun HM et al. Lancet 2004]. However, a meta-analysis of statin use in patients with diabetes reported that the use of intensive-dose statin therapy compared with moderate-dose statin therapy was associated with a slightly higher and dose-dependent incidence of new-onset diabetes [Sattar N et al. Lancet 2010].
Proteinuria is a risk factor for further loss of kidney function and progression to end-stage renal disease in both diabetic and nondiabetic patients. Experimental results have suggested that statins reduce proteinuria and preserve kidney function, but clinical studies have produced mixed results. The two randomized, double-blind, multinational PLANET (Prospective Evaluation of Proteinuria and Renal Function in Non-Diabetic Patients with Progressive Renal Disease) trials tested the effects of atorvastatin 80 mg/day or rosuvastatin 10 or 40 mg/day on urinary protein excretion and renal function in hypercholesterolemic patients with moderate proteinuria. The results suggest that atorvastatin may be protective but that rosuvastatin seems to have no protective effects and in fact may be harmful, particularly in diabetic patients. The question remains whether atorvastatin is actually protecting the kidneys or whether rosuvastatin may impair their function long term.
HDL-C levels play an important and complex role in the development of atherosclerosis. HDL-C contains a small lipid core of cholesteryl esters (CEs) and plasma triglyceride that is surrounded by phospholipids and specialized proteins, known as apolipoproteins (apos). HDL-C and its major apolipoprotein, apoA-I, are synthesized by both the liver and the intestine. Long-term increases in HDL-C levels and reductions in LDL -C levels result from the partial inhibition of cholesteryl ester transfer protein (CETP). Hany Ragy, MD, National Heart Institute, Imbaba, Cairo, Egypt, discussed the antiatherogenic properties of HDL.
There is evidence to suggest a strong relationship between CAD and HDL-C levels. Primary reductions in HDL-C are common in patients with premature CHD, while low HDL-C levels are more common in patients with a first MI than in age-matched controls without CHD. In the Beza fibrate Infarction Prevention (BIP) study, 52% of patients with CHD and with normal LDL-C cholesterol had low HDL-C (<35 mg/dL) [Genest J Jr et al. J Am Coll Cardiol 1992]. The incidence of CHD events in a normal population appears to be inversely related to the serum HDL-C concentration. Data from the Framingham Heart Study show that the risk for MI increases by 25% for every 5-mg/dL decrease in serum HDL-C below the median value. Results from both the LIPID and CARE trials showed that HDL-C levels are also predictive of coronary events in patients with known CHD, especially in the subgroup with LDL-C <125 mg dL. Concentrations of HDL-C >75 mg/dL are associated with longevity and relative freedom from CHD.
Causes of high HDL-C may be primary (ie, genetic) or secondary (eg, cigarette smoking, obesity [particularly visceral fat], and certain drugs). Genetic defects in HDL metabolism or therapeutic interventions that alter HDL metabolism may also affect the development of atherosclerosis. The only known genetic causes of high HDL are deficiencies in the genes for CETP and hepatic lipase. Epidemiological evidence suggests that HDL-C levels are declining over time in the United States (Figure 2).
Declining HDL-C.
Oxford University Press; Evidence to support aggressive management of HDL-cholesterol: implications of recent trials; Taylor AJ; European Heart Journal Supplements 2006; 8 (supp F).
Further elucidation of the molecular mechanisms that are involved in HDL metabolism promises to help identify potential targets for decreasing the incidence and progression of atherosclerotic CV disease. Investigations of the genetic mechanisms that are involved in normal and defective HDL metabolism may lead to the development of novel therapies. Research is focusing on three areas: HDL-associated apolipoproteins; HDL-modifying plasma enzymes and transfer proteins (eg, lecithin, cholesterol acyltransferase, CETP, and hepatic lipase); and cellular and cell surface proteins that are involved in HDL metabolism (eg, ATP-binding cassette transporter 1 and scavenger receptor class-B, type I).
Until these novel approaches are proven to be safe and effective, Prof. Hagy recommends controlling HDL levels with exercise, smoking cessation, a focus on healthy eating, and weight control. “Don't worry too much about your genes now; you can't change that!” he concluded.
Editors Note: Since this presentation, additional important information regarding HDL-targeted therapy has become available. In the spring of 2011, the AIM-HIGH trial (NCT00120289), which randomized patients with vascular disease and atherogenic dyslipidemia to niacin (n=1718) or placebo (n=1696) on a background of LDL-lowering therapy (simvastatin with ability to add ezetimibe, based on achieved LDL [n=515]), was stopped by the National Heart, Lung and Blood Institute (NHLBI) 18 months earlier than planned. In a statement that was posted on the National Institutes of Health website, it was noted that “at a regularly scheduled meeting on April 25, 2011, the study's Data Safety Monitoring Board (DSMB) concluded that high-dose, extended-release niacin offered no benefits beyond statin therapy alone in reducing CV-related complications in this trial. The rate of clinical events was the same in both treatment groups, and there was no evidence that this would change by continuing the trial. For this reason, the DSMB recommended that the NHLBI end the study.”
In addition, a potentially harmful signal was noted: “The DSMB also noted a small and unexplained increase in ischemic stroke rates in the high-dose, extended-release niacin group. This contributed to the NHLBI acting director's decision to stop the trial before its planned conclusion. During the 32-month follow-up period, there were 28 strokes (1.6%) reported during the trial among participants taking high-dose, extended-release niacin versus 12 strokes (0.7%) reported in the control group. Nine of the 28 strokes in the niacin group occurred in participants who had discontinued the drug at least 2 months and up to 4 years before their stroke. Previous studies do not suggest that stroke is a potential complication of niacin, and it remains unclear whether this trend in AIM-HIGH arose by chance [or] was related to niacin administration or some other issue.”
These data will need to be integrated with other findings on outcomes and HDL-targeted therapies. Newer agents that target HDL through alternative mechanisms will also offer information as to whether HDL is solely a marker or risk or whether it is a beneficial therapeutic target.
- © 2011 MD Conference Express