Summary
This article presents results from the recent ABSORB studies, which suggest that bioabsorbable scaffold technology has arrived and has the potential to restore natural vascular integrity and function while having a similar safety profile as a traditional drug-eluting stent.
- interventional techniques & devices
Vessel scaffolding, as can be achieved with an intracoronary stent, may only be needed transiently. Data from experimental, clinical, and pathological studies have suggested that the process of restenosis begins very early after coronary angioplasty, and approximately 3 months after percutaneous transluminal coronary angioplasty, the lumen appears to stabilize [Serruys PW et al. Circulation 1988]. The problem with current stent technology is that the stent remains long after it is no longer needed. The ideal stent would reduce the need for prolonged dual antiplatelet therapy, restore vasomotor function, avoid creating inflammation or vessel irritation, avoid complicating future interventions and reinterventions (particularly in younger patients), avoid interfering with imaging, and disappear when no longer needed. This is the rationale for a bioabsorbable scaffold, the next revolution in stent technology. Bernard Chevalier, MD, Institut Cardiovasculaire Paris Sud, Massy/Quincy, France, presented results from the recent ABSORB studies, which suggest that this technology has arrived and has the potential to restore natural vascular integrity and function while having a similar safety profile as a traditional drug-eluting stent (DES).
The Vascular Everolimus-Eluting Bioresorbable Vascular Scaffold System (BVS) is made of polylactic acid—biodegradable polyester that is derived from lactic acid. This highly crystalline structure provides device integrity and increased radial strength and appears to maintain natural vessel curvature at implantation. It is coated with slow-releasing everolimus at a similar dose density and release rate as the XIENCE V metal stent. The primary mode of degradation is by hydrolysis of ester bonds, which results in a loss of molecular weight but not radial strength, as the strength comes from the crystalline domains. Radial strength of this device is also comparable with XIENCE V. The BVS is made of the same material that is commonly used in medical implants, such as resorbable sutures. The stent is designed to restore blood flow by opening an occluded vessel and providing support until the vessel wall is healed. Once the vessel can remain open without the extra support, the bioresorbable scaffold is designed to metabolize slowly and eventually be absorbed into the arterial wall.
The ABSORB trials were designed to assess the performance of these BVS stents and include Cohort A, which tested the first-generation design; Cohort B, which tested a modified design; and EXTEND, which is ongoing and will evaluate the BVS in a larger population with more complex disease.
In the ABSORB Cohort A trial, 30 stable patients with single, de novo lesions received the new stent and were followed for up to 2 years clinically and with multiple imaging methods. There was no evidence of stent thrombosis after 6 months up to 2 years. The rate of ischemia-driven major adverse cardiac events (MACE) was 3.3%. Late lumen loss occurred at 6 months, mainly due to reduction in scaffold area; very late luminal enlargement was noted from 6 months to 2 years. One MACE occurred in the first 6 months: a non-Q-wave myocardial infarction and one target lesion revascularization in the same patient. There were no additional MACE events between 6 and 24 months.
To help evaluate the reabsorption of the stents in the ABSORB Cohort A trial, optical coherence tomography (OCT) was performed in a BVS-implanted porcine coronary artery model. Stent struts that were still discernible by OCT at 2 years were compatible with largely bioresorbed struts, as demonstrated by histological and gel permeation chromatography analysis. At 3 and 4 years, both OCT and histology confirmed complete integration of the struts into the arterial wall. Mass loss data suggest that 100% of material mass had been lost at 2 years, with no inflammation around the preexisting strut regions. Struts were fully replaced by tissue at 3 years, and by 4 years, sites of preexisting struts were indiscernible.
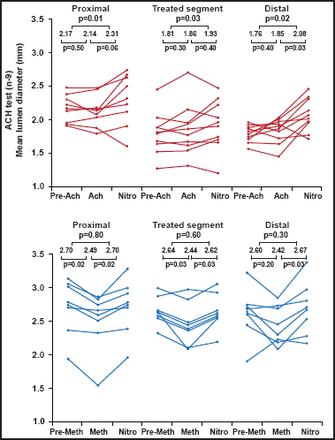
Imaging results in pigs were similar to those noted in patients in the ABSORB Cohort A. At the 2-year angiography, the in-stent late loss of 0.48 mm (SD 0.28) and the diameter stenosis of 27% (11) did not differ from the findings at 6 months. The luminal area enlargement on OCT and intravascular ultrasonography (IVUS) between 6 months and 2 years was due to a decrease in plaque size without change in vessel size. At 2 years, 34.5% of strut locations presented no discernible features by OCT, confirming decreases in echogenicity and in radiofrequency backscattering; the remaining apparent struts were fully apposed. Additionally, vasomotion occurred at the stented site and adjacent coronary artery in response to vasoactive agents, suggesting that a physiological response to vasoactive stimulius might be restored (Figure 1) [Serruys PW et al. Lancet 2009].
Vasomotor Function Testing.
Reproduced with permission from The Lancet; A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods; Serruys PW et al. 2009;373:897–910.
The first generation of the bioresorbable everolimus drug-eluting vascular scaffold that was tested in the ABSORB A trial showed signs of shrinkage at 6 months, which largely contributed to late luminal loss. To maintain the mechanical integrity of the device up to 6 months, the scaffold design and manufacturing process of its polymer were modified to include more uniform strut distribution, more even support of the arterial wall, higher radial strength, lower late stent area loss, room temperature storage capability, and improved device retention.
Using a similar study design, ABSORB B enrolled 101 stable patients to receive this new improved stent. At 1 year, 45 patients successfully received the BVS. One patient had postprocedural release of myocardial enzyme without Q-wave occurrence; 1 patient had OCT-diagnosed disruption of the scaffold, caused by excessive postdilatation. The MACE rate remained consistent, and there were no reports of blood clots. At variance with the ultrasonic changes that were seen with the first-generation BVS device at 6 months, the backscattering of the polymeric struts did not decrease over time. The scaffold area was reduced by only 2.0% with IVUS. OCT showed that 96.8% of the struts were covered and that malapposition of at least 1 strut, initially observed in 12 scaffolds, was detected at follow-up in only 3 scaffolds. The authors concluded that the modifications in these second-generation devices substantially improved the medium-term performance, making it comparable with those of current DES [Serruys PW et al. Circulation 2010].
Four-year data from ABSORB Cohort A continue to be encouraging. The chronic recoil issue that was seen in this first version has been resolved with the version that was used in ABSORB Cohort B, the resorption process is confirmed and is faster in humans compared with pigs, and the restoration of normal vasomotion has been validated. ABSORB Cohort B shows a 12-month performance that is similar to the last-generation DES. The BVS technology permits restoration of natural vascular integrity and function and low incidence of adverse events and thrombosis and should provide unique physiological benefits to patients.
The ABSORB EXTEND trial [NCT01023789] is a single-arm study that is currently enrolling approximately 1000 patients with more complex coronary artery disease at up to 100 centers in Europe, Asia Pacific, Canada, and Latin America. The objective is to extend the assessment of the safety and performance of the BVS.
- © 2011 MD Conference Express