Summary
It is generally accepted that the risk of cardiac events increases as blood pressure increases and that even a small reduction in systolic blood pressure is associated with decreased risk. There is controversy, however, about how aggressively hypertension should be treated. This article reviews several important clinical trials on hypertension.
- Hypertensive Disease
It is generally accepted that the risk of cardiac events increases as blood pressure (BP) increases and that even a small reduction in systolic blood pressure (SBP) is associated with decreased risk. There is controversy, however, about how aggressively (to what target) hypertension should be treated.
To help put the controversy into perspective, Alfred A. Bove, MD, Temple University, Philadelphia, Pennsylvania, USA, reviewed the results from several important clinical trials on hypertension. Among these were ACCORD, which looked at target BP in diabetic patients; NAVIGATOR, which compared the angiotensin II receptor blocker (ARB) valsartan with the glucose-lowering drug nateglinide; and ASCOT, which compared a β-blocker (atenolol) +/− a diuretic with a combination of a calcium channel blocker (CCB; amlodipine) + an angiotensin-converting enzyme (ACE) inhibitor (perindopril). He also reviewed data from INVEST, a study that evaluated treating to tight (<120 mm Hg) versus conventional (<140 mm Hg) BP targets, and HYVET, which looked at treating hypertension in patients aged <80 years. New studies that were discussed included two Japanese studies that looked at treatment of hypertension in diabetics (NAGOYA and OSCAR) and a long-term (20-year) follow-up study (The Bogalusa Heart Study).
In ACCORD, investigators assessed whether targeting SBP to <120 mm Hg reduced cardiovascular (CV) events compared with a strategy that targeted SBP to <140 mm Hg in 4733 patients with type 2 diabetes who were at high risk for CV events. Overall, the trial showed no difference in the primary endpoint of CV events at 1 year (p=0.20). More intensive BP control was also associated with a higher risk of serious adverse events (p<0.001). The annual rate of stroke, a prespecified secondary outcome, was reduced with intensive BP management (in this study, mean achieved BP was ∼118 mm Hg) compared with standard therapy (p=0.01). Achieving this reduction required patients to take multiple medications (>3 in the intensive group), which can be both an economic and compliance factor [ACCORD Study Group. N Engl J Med 2010].
The objective of the NAVIGATOR study was to evaluate whether nateglinide (60 mg 3 times daily) or valsartan (160 mg/day), in a 2-by-2 factorial design, in addition to lifestyle modification reduced the risk of diabetes and CV events compared with placebo in patients with impaired glucose tolerance and either cardiovascular disease (CVD) or risk factors for CVD. A total of 9306 patients were randomized and followed for a median of 5 years. Overall, there was no benefit with nateglinide in terms of a reduction in any of the three coprimary outcomes (development of diabetes, core CV events, and overall CV events). Valsartan therapy led to a 14% relative reduction in the incidence of diabetes (p<0.001) but did not lead to a reduction in either of the CV endpoints in spite of significant (p<0.001) reductions in both SBP (−2.8 mm Hg; 95% CI, 2.4 to 3.2) and diastolic blood pressure (DBP; −1.4 mm Hg; 95% CI, 1.2 to 1.7) [McMurray JJ et al. N Engl J Med 2010].
The NAGOYA Heart Study was a prospective, randomized, open-label study to compare the efficacy of valsartan with amlodipine in reducing CV mortality/morbidity in 1150 hypertensive Japanese patients with glucose intolerance. Target BP was <130/80 mm Hg. The primary endpoint was a composite of CV events (myocardial infarction [MI], stroke, hospitalization due to heart failure [HF], coronary intervention, and sudden cardiac death). Both arms achieved similar BP reductions (mean BP was reduced to 131/73 mm Hg in the valsartan group and 132/74 mm Hg in the amlodipine group) at 54 months. There was no difference in the primary composite outcome; however, valsartan significantly reduced the risk of congestive HF (HR, 0.20; 95% CI, 0.06 to 0.69; p=0.01) [Murohara T et al. ACC 2011].
The OSCAR Study was designed to examine the effect of a high-dose ARB (olmesartan 40 mg) versus an ARB (olmesartan 20 mg) + a CCB (azelnidipine or amlodipine) on CV events and all-cause mortality in elderly Japanese patients with poorly controlled hypertension who were already on standard doses of an ARB. A total of 1164 patients were randomized, and after 3 years of follow-up, there was no difference in the primary composite CV endpoint. In the subgroup of patients with CVD, subjects who were randomized to combination therapy had significantly fewer occurrences of CV events and death than those in the monotherapy group (p=0.0261), whereas patients with type 2 diabetes only appeared to benefit from treatment with the high-dose ARB. Combination treatment was associated with significantly lower BP at 3 years (SBP 2.4 mm Hg lower; p=0.03) [Ogawa H et al. ACC 2011].
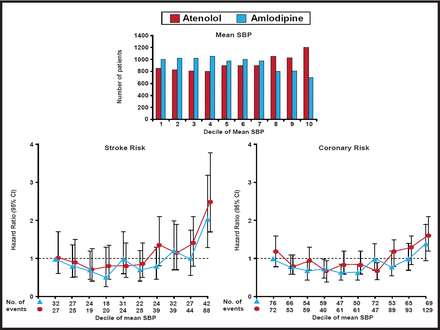
The ASCOT trial, which randomized 19,257 patients with hypertension to either amlodipine with the addition of perindopril if needed or atenolol with the addition of bendroflumethiazide if needed, showed a reduction in all major CV events, all-cause mortality, fatal and nonfatal stroke, and new-onset diabetes with amlodipine/perindopril. In addition, ASCOT showed that visit-to-visit BP variability (an outcome variable) was a powerful predictor of both stroke and CHD (Figure 1). Amlodipine was more effective in lowering BP than atenolol and was also more effective in reducing both stroke and coronary risk at the higher deciles of BP, suggesting that better long-term outcome results can be achieved using a combination of an ACE inhibitor and a CCB than a β-blocker + a thiazide. The authors note, however, that on the basis of prior trial evidence, the effects that were observed might not be entirely explained by better control of BP alone [Dalhöf B et al. Lancet 2005].
ASCOT Trial: BP-Lowering Arm.
The Lancet. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Dahlöf B et al. Jan 1, 2005;366(9489):895–906.
Results of the INVEST trial, which randomized 22,576 patients with hypertension and CAD, showed that compared with usual control (SBP 130 to 140 mm Hg), tight control (SBP<130 mm Hg) was not associated with an improvement in either the primary composite endpoint (nonfatal MI, total MI, nonfatal stroke, or total stroke) or any of the individual components but was associated with an increase in all-cause mortality both during the initial study (at 5 years) and on extended follow-up (out to 11 years). The existence of a U-shaped curve for DBP and the incidence of MI has also been shown, which appears to be between 70 and <90 mm Hg [Messerli FH et al. Ann Intern Med 2006].
HYVET was a study in 3845 patients aged >80 years with an SBP >160 mm Hg, randomized to indapamide with or without perindopril. The primary study outcome was all-cause mortality. After 4 years, participants in the indapamide + perindopril arm achieved a mean SBP of about 148 to 150 mm Hg (vs ∼173 mm Hg at baseline), which was associated with a significant reduction in all-cause mortality (p=0.02), a nonsignificant reduction in stroke, and a significant reduction in stroke mortality (p=0.05). Combination treatment was associated with a significant (p<0.001) reduction in heart failure. An important lesson from this study is that in this population of patients, substantial improvements can be obtained just by getting their SBP into the 150-mm Hg range [Becket NS et al. N Engl J Med 2008].
The Bogalusa Heart Study followed a diverse population (1053 subjects [718 whites; 335 blacks]; 42% men; mean age 38.4 years [range 24 to 48 years]) for an average of 19.7 years. Participants were examined serially 4 to 14 times for BP, and echocardiography was performed in adulthood between 2001 and 2009. BP variability, which was more common among blacks, was shown to correlate with left ventricular hypertrophy (r=0.66; p<0.001), which correlates with poor long-term outcome [Chen W et al. ACC 2010].
In summary, Dr. Bove reviewed specific guidelines and RCT-based recommended therapies for a variety of conditions and target BPs (Table 1).
Specific Drug Indications.
- © 2011 MD Conference Express