Summary
This article reviewes two new techniques that are under development for percutaneous repair of the mitral valve.
- valvular disease
- inflammatory disease
- interventional techniques & devices
Mitral regurgitation (MR), the most common type of heart valve insufficiency, affects more than 4 million people in the United States [Nkomo VT et al. Lancet 2006]. The volume overload that is associated with MR and heart failure (HF) contributes to ventricular remodeling and, over time, may lead to irregular heartbeat, HF, stroke, heart attack, or death. Dilated cardiomyopathy is characterized by significant enlargement of cardiac chambers, which can lead to functional mitral regurgitation (FMR), which increases the risk of morbidity and mortality even further. Horst Sievert, MD, CardioVascular Center Frankfurt, Frankfurt, Germany, reviewed two new techniques that are under development for percutaneous repair of the mitral valve.
The CARILLON Mitral Contour System™ is a nonsurgical, minimally invasive device that is designed to repair the mitral valve and reduce FMR. It combines a proprietary implantable device and a percutaneous delivery system. The procedure starts with a venogram to characterize anatomy, then placement of a distal anchor near the anterior commissure; tension is applied to plicate the tissue to reduce MR. If a good position and reduction in MR are confirmed, the device is released. The efficacy and safety of the CARILLON system in FMR were evaluated in the Phase I TITAN trial of 53 patients with dilated ischemic or nonischemic cardiomyopathy (LVEDd >55 mm). Implantation was successful in 68% (36/53) of patients. Treatment with the system was associated with an average 40% reduction in echocardiography core lab-derived quantitative measures of FMR over a period of 12 months. Six-minute walk distance and NYHA Class also improved (Table 1). There were no device-related major adverse events (AEs) at 12 months. Mortality in the implanted and nonimplanted groups was similar at 1 year [Siminiak T et al. ESC 2010].
Functional Changes.
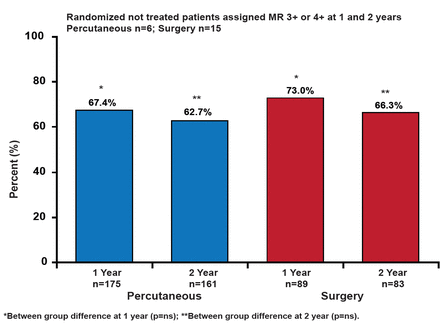
The MitraClip® System is a catheter-based therapy that is adapted from the open surgical double-orifice technique. The system is intended to be an additional option for patients who are suitable for a percutaneous approach. It consists of three major subsystems: a steerable guide catheter, a clip delivery system, and the MitraClip device (implant). The EVEREST II (Endovascular Valve Edge-to-Edge Repair Study) trial compared percutaneous mitral valve repair using the MitraClip system with surgical repair or replacement in 279 patients with moderate/severe (3+) or severe (4+) MR who were candidates for mitral valve (MV) surgery. The primary efficacy endpoint was freedom from death, MV surgery/reoperation, or grade 3+ or 4+ MR at 12 months. The primary safety endpoint was a composite of major AEs within 30 days. Through 2 years, there has been no device embolization, fracture, erosion, or migration [Feldman T et al. N Engl J Med 2011]. No additional occurrence of single leaflet device attachment occurred between 1 and 2 years (the 1-year rate was 6.3%). At Year 2, in an intent-to-treat analysis, significantly more patients in the surgical group met the primary efficacy endpoint (66.3% vs 51.7%; p=0.04). However, the difference was not significant when using a comparison of treatment strategy analysis (62.7% and 66.3%, percutaneous and surgery, respectively; p=NS; Figure 1). Patients who underwent percutaneous MV repair had significant improvements in left ventricular (LV) end systolic/diastolic volume and NYHA functional classification compared with patients who had surgery (p<0.005 in all comparisons).
Primary Effectiveness Analyses at 1 and 2 Years.
Reproduced with permission from H. Sievert, MD.
Although surgery provided more complete MR reduction, percutaneous repair was associated with increased safety, improved LV dimensions, and clinical improvements in NYHA class and quality of life. The system's positive risk-benefit profile supports its use as a treatment for patients who are not good candidates for surgery and have few other options, including elderly or frail patients and those who are at high risk for surgery.
- © 2011 MD Conference Express