Summary
Prior treatment with atorvastatin is associated with a reduction in the risk of all-cause mortality compared with placebo 8 years after the early termination of the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm [ASCOT-LLA] and 11 years after initial randomization, according to new findings from a long-term follow-up study.
- Hypertensive Disease
- Lipid Disorders Clinical Trials
Prior treatment with atorvastatin is associated with a reduction in the risk of all-cause mortality compared with placebo 8 years after the early termination of the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA) and 11 years after initial randomization, according to new findings from a long-term follow-up study.
In 2003, an interim analysis of ASCOT-LLA showed that atorvastatin significantly reduced the risk of coronary heart disease (CHD; RRR 36%) and stroke (RRR 27%) compared with placebo in patients with hypertension who were also receiving antihypertensive treatment, leading to an early termination of the trial [Sever PS et al. Lancet 2003]. The subgroup of patients who were enrolled in the United Kingdom (UK) cohort of ASCOT-LLA was then followed for an additional 8 years after trial termination on open-label therapy, as selected by the local health care provider. Peter S. Server, MD, FRCP, Imperial College, London, UK, presented mortality results for the entire 11-year follow-up period since initial randomization in ASCOT-LLA.
In the ASCOT-LLA randomized trial, 10,305 patients with hypertension and a total cholesterol level of ≤6.5 mmol/L (250 mg/dL) were randomly assigned to atorvastatin 10 mg or placebo. After a median follow-up of 3.3 years, the trial was terminated due to overwhelming benefit with atorvastatin, with a reduction in the primary endpoint of nonfatal myocardial infarction (MI) and fatal CHD of 36% compared with placebo (HR, 0.64; 95% CI, 0.50 to 0.83; p=0.0005). At that time, there was no significant difference between groups in terms of either all-cause mortality (HR, 0.87; 95% CI, 0.71 to 1.06) or cardiovascular (CV) mortality (HR, 0.90; 95% CI, 0.66 to 1.23).
After ASCOT-LLA was terminated, investigators continued to collect mortality data in the UK cohort (n=4605) for a total median follow-up of 11 years from initial randomization. Mortality data were available from the UK Office for National Statistics and General Register Office for Scotland, and the cause of death was identified in death certificates.
By the end of the ASCOT-LLA extension study, most patients who were initially randomized to atorvastatin therapy continued to take atorvastatin (63%), while a minority (4%) took another statin. Likewise, most patients in the placebo group also switched to atorvastatin (56%), with a small number initiating therapy with another statin (7%).
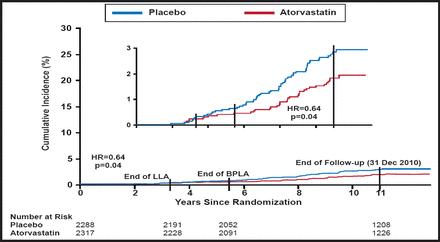
Through 11 years of median follow-up in the ASCOT-LLA extension group, the risk of all-cause mortality was 14% lower for those who were initially assigned to atorvastatin compared with placebo (HR, 0.86; 95% CI, 0.76 to 0.98; p=0.02). The survival benefit was driven by a reduction in non-CV deaths (HR, 0.85; 95% CI, 0.73 to 0.99; p=0.03). In particular, patients who were initially randomized to atorvastatin had a lower long-term risk of death due to infections and respiratory illness (HR, 0.64; 95% CI, 0.42 to 0.97; p=0.04; Figure 1). By comparison, there was no difference in the risk of death due to CV causes (HR, 0.89; 95% CI, 0.72 to 1.11; p=0.32).
Cumulative Incidence of Mortality Due to Combined Infection and Respiratory Disease.
Reproduced with permission from Oxford University Press. The Anglo-Scandinavian Cardiac Outcomes Trial: 11-year mortality follow-up of the lipid-lowering arm in the UK. Sever PS et al. Eur Heart J. 28 Aug 2011.
These observations from the ASCOT-LLA extension study may suggest a legacy benefit in terms of a reduction in mortality with atorvastatin, further underscoring the benefit of statins. The mechanism by which statin therapy may reduce the risk of infection and non-CV death over the long term is unclear and needs verification in additional studies, given the limitations of this nonrandomized comparison. Future prospective studies may determine if statins can reduce the risk of sepsis or death from infectious illness, Prof. Sever concluded.
- © 2011 MD Conference Express