Summary
This overview presents progress in bone biology and highlighted potential new targets for therapeutic intervention. Included are the basic anatomy and physiology of bone; recent progress in the comprehension of the processes of bone formation and degradation; and the potential of novel therapeutic agents for bone disease in arthritis and osteoporosis.
- arthritis
- metabolic bone disease
Dallas C. Jones, PhD, Harvard Medical School, Boston, Massachusetts, USA, provided an overview of progress in bone biology and highlighted potential new targets for therapeutic intervention. His presentation included the basic anatomy and physiology of bone; recent progress in the comprehension of the processes of bone formation and degradation; and the potential of novel therapeutic agents for bone disease in arthritis and osteoporosis.
The skeleton is an elaborate structure made of bones and cartilage, articulating with one another to serve important mechanical, metabolic, and microenvironmental functions. These include locomotion, the protection of vital organs, lodging of hematopoiesis, and mineral homeostasis [Teti A. Curr Osteoporos Rep 2011].
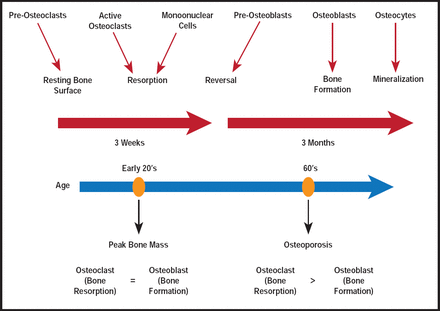
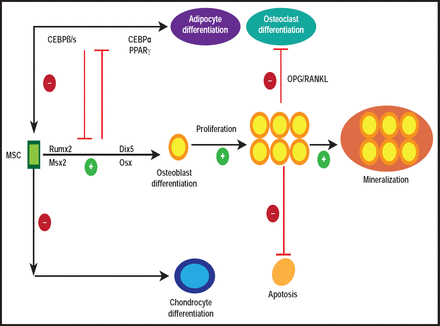
Remodeling is a highly balanced process that involves the orchestration of bone cell activities (Figure 1). It involves the removal of old or damaged bone by osteoclasts (ie, bone-resorbing cells) and the subsequent replacement by new bone, formed by osteoblasts (ie, bone-forming cells; Figure 2) [Feng X, McDonald JM. Annu Rev Pathol Mech Dis 2011].
Bone Remodeling and Outcomes.
Reproduced with permission from Annual Reviews Inc. Copyright © 2010. All rights reserved.
Osteoblast Basics.
Reproduced with permission from Annual Reviews Inc. Copyright © 2010. All rights reserved.
To prevent alterations in bone mass or quality after each remodeling cycle, bone resorption and formation must be tightly coupled. However, a variety of factors (eg, menopause-associated hormonal changes, drugs, age-related factors) can derail the process. Disequilibrium, in turn, leads to dysfunctions that can be seen in several bone diseases, including osteoporosis [Robling AG, Turner CH. Crit Rev Eukaryot Gene Expr 2009; Feng X, McDonald JM. Annu Rev Pathol 2011].
Osteoporosis is a major public health issue that will only get worse as the population ages. In the United States, an estimated 55% of those aged ≥50 years are at risk for osteporotic fractures. In 2025, more than 3 million osteoporotic fractures are expected, with associated costs rising to approximately $25.3 billion [National Osteoporosis Foundation. www.nof.org 2011].
Anticatabolic Therapeutics
Anticatabolic therapeutic interventions target resorptive and anabolic events. The former includes biphosphonates (BPs), cathepsin K inhibitors, and receptor activator of NF-κB ligand (RANKL) inhibitors [Luhmann T et al. J Control Release 2011]; the latter includes Wnt/LRP5, Eph/Ephrins, Sema4D/PlexinB1, and Schnurri-3. During his presentation, Dr. Jones discussed current and emerging therapeutics.
BPs reduce osteoclastic activity through inhibition of farnesyl diphosphate synthase, which leads to a loss in guanosine triphosphate (GTP)-binding proteins. These proteins are the key to osteoclastic activity, and it is the interference within the mevalonate pathway that stops osteoclastic activity and bone resorption [Russell RG et al. Ann NY Acad Sci 2007].
RANKL, a member of the tumor necrosis factor family, is pivotal in osteoclastogenesis, as well as in mature osteoclast activity [Luhmann T et al. J Control Release 2011]. Recent data suggest that osteocytes are the major source of RANKL in bone remodeling in vivo [Nakashima T et al. Nat Med 2011]. Denosumab, a fully human monoclonal antibody, acts by binding to and inhibiting RANKL, leading to the loss of osteoclasts from bone surfaces [Baron R et al. Bone 2011].
Cathepsin K, a lysomal cysteine protease that is involved in osteoclast-mediated bone resorption and inhibition, is a potentially attractive therapeutic approach for treating diseases that are characterized by excessive bone resorption [Wijkmans J, Gossen J. Expert Opin Ther Pat 2011]. Most compounds are peptide-derived inhibitors that display a reversible binding nitrile or ketone warhead. Their clinical success will be determined by the selectivity that can be achieved against other off-target cathepsins. Current Phase 2 and Phase 3 clinical trials of ONO-5334 and odanacatib, respectively, may determine the future of these agents as disease-modifying therapeutics [Wijkmans J, Gossen J. Expert Opin Ther Pat 2011].
Anabolic Therapeutics
The Lrp5 gene in the wnt pathway is a major determinant of bone mass accrual. Data indicate that circulating serotonin levels mediate the increased bone mass that result from gain-of-function mutations in Lrp5 in humans [Frost M. et al. J Bone Miner Res 2010]. Lrp5 signaling functions locally, suggesting that increasing Lrp5 signaling in mature bone cells may be a strategy for treating human disorders that are associated with low bone mass [Cui Y et al. Nat Med 2011].
Sclerostin is secreted by the osteocyte network and preosteoclasts and binds to the Lrp5/6 receptors on osteoblasts to inhibit wnt signaling. Preclinical results suggest that sclerostin is a pivotal negative regulator of bone formation in the aging skeleton [Li X et al. J Bone Miner Res 2009]. Sclerostin antibodies (AMG 785) are currently in Phase 2 development and have been reported to be well tolerated in Phase 1 trials [Padhi D et al. J Bone Miner 2011].
Osteoclasts express the NFATc1 target gene Efnb2 (encoding ephrinB2), while osteoblasts express the receptor EphB4, along with other ephrin-Eph family members. Gain- and loss-of-function experiments demonstrate that reverse signaling through ephrinB2 in osteoclast precursors suppresses osteoclast differentiation by inhibiting the osteoclastogenic c-Fos-NFATc1 cascade.
In addition, forward signaling through EphB4 to osteoblasts enhances osteogenic differentiation, and overexpression of EphB4 in osteoblasts increases bone mass in transgenic mice. These data demonstrate that ephrin-Eph bidirectional signaling links two major molecular mechanisms for cell differentiation—one in osteoclasts and the other in osteoblasts—thereby maintaining bone homeostasis [Zhao C et al. Cell Metab 2006].
Sema4D, an axon guidance molecule that potentially inhibits bone formation, has emerged as a new therapeutic target for the discovery and development of bone-increasing drugs. Binding of Sema4D to its receptor, Plexin-B1, on osteoblasts results in the activation of the small GTPase RhoA, which inhibits bone formation by suppressing insulin-like growth factor-1 signaling and by modulating osteoblast motility [Negishi-Koga T et al. Nat Med 2011].
Initial in vitro studies report various functions for mammalian Schnurri (Shn) proteins. Mice that bear parallel null mutations in the adapter proteins Shn2 and Shn3 exhibit defects in patterning of the axial skeleton during embryogenesis. Postnatally, these compound mutant mice develop unique osteochondrodysplasia.
The deletion of Shn 2 and Shn3 impairs growth plate maturation during endochondrial ossification but simultaneously results in massively elevated trabecular bone formation. These findings indicate that growth plate maturation and bone formation can be uncoupled under certain circumstances and that unique and redundant functions that reside in the Schnurri protein family are required for proper skeletal patterning and remodeling [Jones DC et al. PNAS 2010].
- © 2011 MD Conference Express