Summary
Cases of multidrug-resistant tuberculosis (MDR-TB) are increasing. MDR complicates therapy and results in lower success rates and higher mortality, especially in HIV coinfected patients. This article discusses the conundrum of MDR-TB and combination therapy and ways to address it.
- Bacterial Infections
- HIV & AIDS
Cases of multidrug-resistant tuberculosis (MDR-TB), defined as TB that is resistant to isoniazid (INH) and rifampin (RMP) and extremely (or extensively) drug-resistant TB [ie, TB that is not only resistant to INH/RMP, but also to quinolone and capreomycin (cyclic peptide) or an aminoglycoside (amikacin or kanamycin)], are increasing. MDR complicates therapy and results in lower success rates and higher mortality, especially in HIV coinfected patients. George L. Drusano, MD, University of Florida, Gainesville, Florida, USA, discussed the conundrum of MDR-TB and combination therapy and ways to address it.
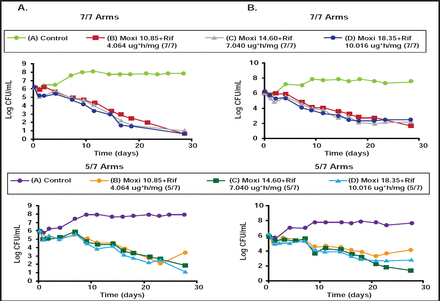
According to Dr. Drusano, combination therapy generally suppresses resistance (Table 1). [Drusano GL et al. mBio 2010]. However, when drugs have vastly different half-lives (eg, rifampin and moxifloxacin) and one induces error-prone replication (as with moxifloxacin), resistance can develop with drug holidays (Figure 1) [Drusano GL et al. mBio 2011]. “If we wish to shorten therapy,” he said, “we have to suppress resistance, pay attention to schedule, and find combinations that are not only antagonistic but, hopefully, synergistic.”
Resistance Suppression: Log-Phase.
Resistance Emergence in Drug Holidays.
Moxi=moxifloxacin; Rif=rifampin.
Reproduced with permission from G. Drusano, MD.
For the first time in decades, new TB drugs are available. Dr. Drusano stressed the importance of not risking the emergence of resistance to these agents and questioned the use of a study design in which these drugs are being evaluated against MDR-TB in an optimized background. Lack of knowledge of the detailed susceptibility pattern of the isolate prior to initiation of therapy increases the risk of creating resistant mutants. The question then becomes how to test the drugs in clinical trials.
He suggested starting by examining INH, to which the bug must be resistant, to be called MDR-TB. INH kills rapidly, with multilog decline at standard drug exposures with low minimal inhibitory concentration (MIC) values. Doses as large as 1200 mg cannot completely counter-select resistance amplification as monotherapy. Area under the curve/MIC is the pharmacodynamic-linked variable for cell kill. Because of the fast/slow acetylator divide, different populations will respond somewhat differently to INH. Specifically, fast acetylators will obtain suboptimal results at MIC values >0.25 mg/L. Therefore, noted Dr. Drusano, “wouldn't it be nice to know the MIC distributions in different areas of the world?” Unfortunately, such a database does not exist. In the absence of that information, Dr. Drusano noted that he would test the new drugs in standard patients to learn how to optimize them in combination and to suppress resistance. He stressed the risk of generating and spreading a number of resistant isolates if new drugs are used to treat MDR- and extremely drug-resistent-TB patients as single drugs.
“Combination therapy is the key for successful treatment of TB,” he said. He stressed the need to learn to use new agents alone and, more importantly, in combination to optimally kill organisms and suppress resistance. He also suggested a dialog with regulatory officials to get these issues out on the table.
The United States Food and Drug Administration's (FDA) Critical Path Initiative is already transforming the way FDA-regulated products are developed, evaluated, and manufactured. One way is by publishing articles on models and approaches for anti-TB drug testing. “The Global Alliance is taking similar actions,” said Dr. Drusano.
- © 2011 MD Conference Express