Summary
This article discusses studies that have shown the effectiveness of new and potential antiretroviral therapy therapies for the treatment of HIV infection, including single-tablet regimens, coformulations, nucleoside reverse transcriptase inhibitor-sparing regimens, CCR5 antagonists as initial therapy, and new entry inhibitors.
- Sexually Transmitted Diseases
- Emerging Therapies
- HIV & AIDS
Improvements in antiretroviral therapy (ART) have made the treatment of HIV infection more potent and better tolerated. While current treatment regimens still have limitations, they are more effective, more convenient, and less toxic than those that were used in the early ART era. Joel E. Gallant, MD, MPH, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA, discussed studies that have shown the effectiveness of new and potential ART therapies, including single-tablet regimens, coformulations, nucleoside reverse transcriptase inhibitor (NRTI)-sparing regimens, CCR5 antagonists as initial therapy, and new entry inhibitors.
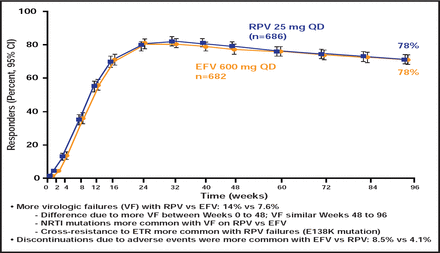
ECHO [NCT00540449] and THRIVE [NCT00543725], two randomized Phase 3 trials, showed that the recently approved non-nucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine (RPV) has sustained efficacy that is noninferior to efavirenz (EFV) in ART-naïve adults who are infected with HIV-1 [Cohen CJ et al. Lancet 2011; Molina JM et al. Lancet 2011]. There were fewer discontinuations that were due to adverse events and fewer treatment-limiting side effects (especially neurological and dermatological) in the RPV arm but more virological failure and resistance compared with the EFV arm, most notably in participants with baseline viral loads >100,000 copies/mL (Figure 1). RPV has been approved both as a single agent and in a coformulation with tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC). It is taken once daily with a meal and is contraindicated in patients who are taking proton pump inhibitors.
ECHO/THRIVE Outcomes.
Reproduced with permission from The Lancet. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Molina JM et al. July 16, 2011;378(9787)238–246.
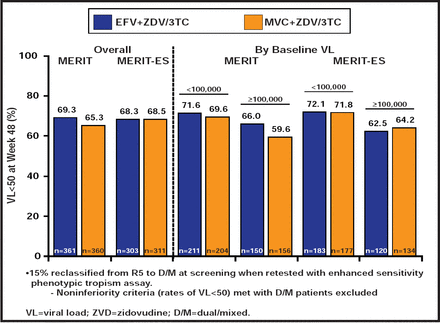
In the MERIT study [NCT00098293], maraviroc (MVC) BID was not noninferior to EFV at <50 copies/mL in the primary analysis in ART-naïve patients with CCR5-tropic virus. However, 15% of patients in the original MERIT trial had dual/mixed-tropic virus, using the more sensitive tropism assay. After exclusion of data from those patients, the MVC arm met noninferiority criteria compared with EFV [Cooper DA et al. J Infect Dis 2010] (Figure 2). QD administration of MVC is also being studied. In a post hoc analysis from the original MOTIVATE trials, which initially included a QD MVC arm, virological suppression was comparable in patients who were treated with MVC QD and BID. MVC has been approved for initial therapy. Potential advantages include its excellent tolerability, its high barrier to resistance, and the fact that treatment-naïve patients are more likely to have CCR5-tropic virus than treatment-experienced patients. The main disadvantage is the need for baseline tropism testing.
Virological and Immunological Outcomes.
Reproduced with permission from JE Gallant, MD, MPH.
A variety of “nucleoside-sparing regimens” have been studied in clinical trials, though none to date has demonstrated sufficient efficacy and/or tolerability to make it a standard-of-care regimen. Examples that have been studied to date include combinations of a boosted protease inhibitor plus EFV, raltegravir (RAL), or MVC.
Elvitegravir (EVG) is an investigational integrase inhibitor that requires pharmacological “boosting” by either ritonavir (RT) or cobicistat (COBI), an experimental pharmacokinetic enhancer, or “booster.” Phase 2 data suggested that a ‘quad’ regimen of once-daily EVG/COBI/FTC/tenofovir disoproxil fumarate (TDF) achieves and maintains a high rate of virological suppression with fewer central nervous system and psychiatric adverse effects compared with the current standard-of-care regimen (EFV/FTC/TDF) [Cohen C. AIDS 2011], and a similarly designed Phase 3 study apparently shows noninferiority of the “quad” compared with EFV, with similar discontinuation rates due to adverse events in both arms [Gilead press release. August 15, 2011]. A study that compared EVG with raltegravir (RAL) in treatment-experienced patients found that EVG was noninferior to RAL [Molina JM. IAS 2011 Rome]. COBI is also being studied as a booster for protease inhibitors. In a Phase 2 trial, the efficacy of a COBI-boosted atazanavir (ATV)-based regimen was similar to that of a RT-boosted ATV-based regimen [Cohen C. AIDS 2011]. Cobicistat is associated with a modest increase in serum creatinine, with a resulting decrease in estimated glomerular filtration rate (GFR), but not measured GFR. This appears to be due to its effect on creatinine transport by the renal tubules rather than to true nephrotoxicity [German P. ICAAC 2011; Lepist EI. ICAAC 2011].
Dolutegravir (DTG) is another promising integrase inhibitor. In the SPRING-1 trial, which was conducted in ART-naïve patients, it was noninferior to EFV with better tolerability [van Lunzen J. IAS 2011 Rome]. There was no selection of integrase mutations in patients who failed, and tolerability was better than with EFV. As with COBI, DTG decreases estimated GFR but not actual GFR, by a mechanism that is similar to that of COBI [Koteff J et al. ICAAC 2011]. DTG may have some activity against RAL- or EVG-resistant virus, especially when dosed twice daily [Eron J. CROI 2011].
GS-7340 is a new tenofovir prodrug that achieves higher intracellular tenofovir levels with lower plasma levels compared with TDF [Markowitz M. CROI 2011]. The hope is that it will be more potent than TDF at smaller doses with less nephrotoxicity.
Lersivirine (LRV) is an investigational NNRTI that had overall efficacy that was similar to that of EFV in a Phase 2 study of treatment-naïve patients [Pozniak A. IAS 2011]. Efficacy was lower in patients with viral loads >100,000 copies/mL. The incidence of grade 3 and 4 adverse events was higher in the EFV arm, although nausea and headache were common with LRV.
There are a number of potential HIV entry inhibitors that can act at various stages of HIV development, such as coreceptor binding, and virus-cell fusion. They include BMS-663068, an oral HIV attachment inhibitor; ibalizumab, an HIV-neutralizing monoclonal antibody; and cenicriviroc, a CCR5 antagonist with anti-CCR2 activity.
- © 2011 MD Conference Express