Summary
Peripheral artery disease (PAD) symptom severity is best described as a functional problem (walking speed and distance, oxygen consumption, level of physical activity, and quality of life). This article discusses claudication therapy, critical limb ischemia, revascularization strategies, treatment options for PAD, and a cost analysis of this disease.
- Prevention & Screening
- Hypertensive Disease
Peripheral artery disease (PAD) symptom severity is best described as a functional problem (walking speed and distance, oxygen consumption, level of physical activity, and quality of life; Table 1). William Hiatt, MD, University of Colorado, Aurora, Colorado, USA, gave an overview of the current state of claudication therapy. Dr. Hiatt noted that while exercise in a supervised setting and in selected patients is effective, the results may not be generalizable, and clear clinical benefit in a broad community setting is lacking. Several therapeutic approaches, such as lipid modulation, antibiotic therapy, and gene therapy, have been tried but were unsuccessful; however, PDE-3 inhibitors have shown modest efficacy. A meta-analysis of nine randomized, controlled trials that used the PDE-3 inhibitor cilostazol to treat claudication in patients showed that cilostazol was associated with a 50.7% improvement from baseline in mean walking distance versus placebo (24.3%). The absolute improvement was 42.1 meters greater than the improvement with placebo (p<0.001) over a mean follow-up period of 20.4 weeks. Continued increases were demonstrated over the 24-week treatment period. Improvements in pain-free walking distance were demonstrable as well [Pande RI et al. Vasc Med 2010].
PAD Symptom Severity.
The overall prevalence of PAD in the United States has been estimated at 7.2% [Allison MA et al. Am J Prev Med 2007]. This translates to about 8.5 million Americans, or roughly one in 16 individuals over the age of 40 years. The risks are significantly greater for African-Americans and Hispanics. Susan Duval, PhD, University of Minnesota, Minneapolis, Minnesota, USA, discussed a ‘nomogram’ that she and her colleagues have developed for estimating PAD probability. The system is based on data from the REACH Registry and is based on age, sex, race, diabetes status, body mass index, hypertension status, smoking status, and coronary artery disease, cardiovascular disease, and congestive heart failure status. Although the scientific knowledge exists to diagnose, treat, and even prevent PAD, awareness of PAD remains low.
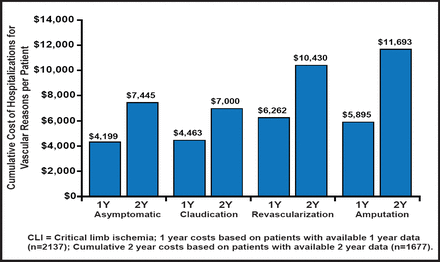
PAD is also expensive. Mean cumulative hospitalization costs, per patient, are estimated to be $7445, $7000, $10,430, and $11,693 for patients with asymptomatic PAD, a history of claudication, lower limb amputation, and revascularization, respectively (p=0.007) [Mahoney EM et al. Circ Cardiovasc Qual Outcomes 2010]. It is estimated that the total annual costs that are associated with vascular hospitalization in PAD patients exceed $21 billion (Figure 1) [Hirsch At et al. Cir Cardiovasc Qual Outcomes 2008]. It is suspected that a significant number of patients with PAD remain undiagnosed. In addition, in patients with PAD, there is underutilization of proven secondary prevention therapies [Pande RL et al. Circulation 2011].
One- and Cumulative 2-Year Costs Associated With Hospitalizations for Vascular Reasons, per Patient, by Baseline PAD Class.
Copyright © 2011 American Heart Association. All rights reserved.
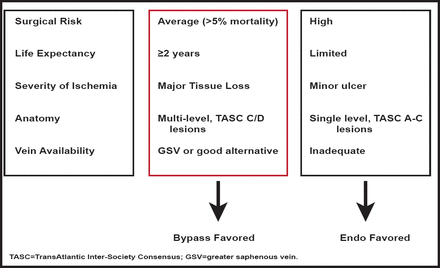
Critical limb ischemia (CLI) is defined as ischemia rest pain or tissue loss and compromised leg hemodynamics and has a high amputation and mortality rate. Therapies are limited, and no proven treatment targets have been identified. Michael S. Conte, MD, Brigham and Women's, Boston, Massachusetts, USA, discussed the significant issues that are associated with diagnosis and management of CLI.
Revascularization is effective for wound healing, functional limb preservation, and pain relief. One small study in a highly selected group of diabetic patients with CLI reported that revascularization allows the postponement of major amputation and improves survival rates compared with nonrevascularized amputated patients (Figure 2) [Faglia E. J Diab Comp 2010].
CLI: A Selective Revascularization Strategy.
Reproduced with permission from MS Conte, MD
A relatively new and evolving approach is endovascular therapy for limb salvage. It offers the potential advantages of being less invasive with faster recoveries; however, data that have evaluated outcomes with this therapy are mixed. Disadvantages include reduced efficacy compared with existing therapy in terms of hemodynamics and durability, risk of limb deterioration, possible effect on surgical options, and increased treatment cost due to the need for repeat treatments. A meta-analysis of studies in infrapopliteal angioplasty compared with bypass surgery for chronic CLI showed limited technical success and durability with angioplasty but comparable limb salvage rates [Romiti M et al. J Vasc Surg 2008]. However, another study [Vogel TR et al. J Vasc Surg 2011] reported higher rates of in-hospital complications, rehospitalization, and amputations with angioplasty. A third study reported slightly lower mortality rates and increased costs with angioplasty treatment [Sachs T et al. J Vasc Surg 2011]. Longitudinal studies are recommended to determine the appropriateness of angioplasty in both claudication and limb-threatened patients.
Surgical bypass of peripheral arterial occlusive disease with autologous vein grafts provides an effective means of restoring blood flow to the lower extremity and has been a standard therapy for patients with disabling claudication or CLI. However, failure rates may run as high as 50% within 5 years. Further, prior failed endovascular interventions in patients with CLI predict poor outcomes after lower extremity bypass. Dr. Conti predicts that centers for amputation prevention will focus on high-risk patients and provide coordinated inpatient and outpatient management. They will offer a multidisciplinary team approach, with vascular surgery and podiatry being key players.
Treatment options for symptomatic PAD are limited, and while medical therapies may help to modify the underlying disease, there are no approved medical (nonrevascularization) therapies to increase blood flow to the ischemic limb. Brian Annex, MD, University of Virginia, Charlottesville, Virginia, USA, discussed genetic and cell-based studies that aim to improve limb perfusion in patients with PAD.
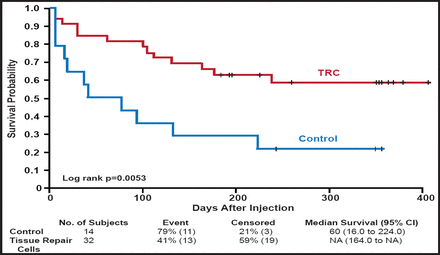
In a 2008 study that comprised 104 patients with CLI, Powell and colleagues demonstrated that intramuscular injection of high doses (4.0 mg at Days 0, 14, and 28) of hepatocyte growth factor (HGF) plasmid was safe and led to significant increases in transcutaneous oxygen tension (TcPo2; 24.0±4.2 mm Hg) compared with placebo (9.4± 4.2 mm Hg), low dose (0.4 mg at Days 0, 14, and 28; 11.7± 3.7 mm Hg), and middle dose (4.0 mg at Days 0 and 28; 7.3±4.8 mm Hg) HGF plasmid (ANCOVA p=0.0015) [Powell RJ et al. Circulation 2008]. Recently, the same group showed that this therapy can also improve toe brachial index (0.05±0.05 vs −0.17±0.04 for placebo; p=0.047) and rest pain, as assessed using a 10-cm visual analog scale (VAS; −1.9±1.3 vs +0.06±0.2 for placebo; p=0.04) [Powell R et al. J Vasc Surg 2010]. Early bone marrow studies showed that autologous implantation of bone marrow mononuclear cells in patients with PAD-induced ischemia produced improvements in ankle brachial index, transcutaneous oxygen pressure, rest pain, and pain-free walking time [Tateishi-Yuyama E et al. Lancet 2002]. More recently, Powell and colleagues evaluated patients with CLI and showed that injection of tissue repair cells led to improvements in time to treatment failure, defined as amputation-free survival, increase in wound size, and new gangrene (Figure 3) [Powell et al. J Vasc Surg 2011].
Time to First Occurrence of Treatment Failure (ITT Population- All Patients).
Reprinted from the Journal of Vascular Surgery, Vol. 54, Issue 4, Pages 1032–1041, Powell RJ et al, Interim analysis results from the RESTORE-CLI, a randomized, double-blind, multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia, Copyright 2011, with permission from Elsevier.
A recent CLI study used autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells [Perin EC et al. Cath Card Int 2011] and another recently completed clinical trial evaluated the use of autologous CD34+ cells for CLI [NCT00616980]. While these early studies show promise for the development of noninterventional therapies to improve limb perfusion, further studies are required to determine whether they are useful in current clinical care.
- © 2011 MD Conference Express®